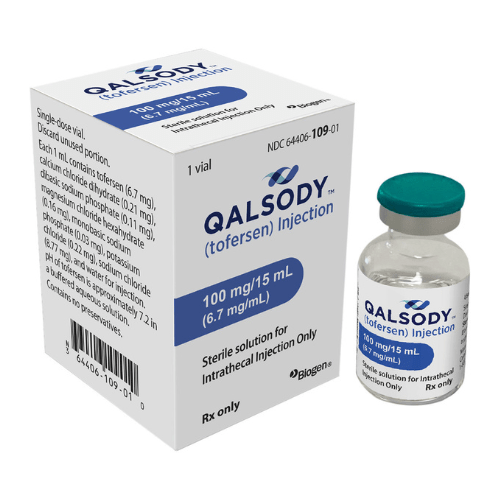
About Qalsody
- Qalsody (Tofersen) belongs to the pharmacological family called Antisense Oligonucleotides.
- Qalsody (Tofersen) is a medication for adults with a specific genetic mutation in the SOD1 gene, aimed at treating amyotrophic lateral sclerosis (ALS).
- It is the first FDA-approved therapy designed to target the genetic source of ALS and slow down its progression.
- Qalsody functions by reducing the levels of the harmful SOD1 protein, which is responsible for damaging motor neurons and causing ALS symptoms.
- Individuals with ALS linked to an SOD1 gene mutation generate a detrimental, misfolded version of the SOD1 protein, resulting in motor neuron damage, muscle weakness, loss of function, and, ultimately, fatality.
- Qalsody received FDA approval for the treatment of amyotrophic lateral sclerosis (ALS) on June 7, 2022.
- This approval is significant as it offers a novel approach to slow the progression of ALS by addressing the underlying genetic mutation.
Strength:
Injection: The injection is available in single-dose vials, each containing a 100 mg/15 mL (6.7 mg/mL) solution.
Recommended Dosage:
Qalsody injection should be administered intrathecally through a lumbar puncture by healthcare professionals skilled in this procedure.
- The recommended dose for Qalsody is 100 milligrams (15 mL) per administration. To initiate the treatment, three loading doses are administered at 14-day intervals.
- Following the loading doses, a maintenance dose of Qalsody injection should be administered once every 28 days to effectively manage amyotrophic lateral sclerosis (ALS) in individuals with a specific genetic mutation in the SOD1 gene.
- In case the second loading dose is skipped, it should be given at the earliest opportunity, with the third loading dose administered 14 days later. If the third loading dose or any subsequent maintenance dose is missed, it should be administered promptly, followed by the next dose after 28 days.
- This treatment regimen is designed to slow the progression of the disease and enhance the quality of life for those affected by ALS.
Important
Qalsody preparation and administration are precise procedures. The vial should reach room temperature without external heat, and clarity should be verified, avoiding shaking. Patient sedation or imaging may be needed. Ensure patient readiness before opening the vial. A lumbar puncture needle removes 10 mL of cerebrospinal fluid.
Administer Qalsody by withdrawing 15 mL (equivalent to 100 mg) from the vial using a needle, with no dilution or need for external filters. The intrathecal injection should be promptly performed, as Qalsody lacks preservatives. Discard unused vial contents to maintain procedure integrity.
Warnings & Precautions
- Qalsody has been associated with serious adverse reactions, including myelitis and radiculitis in six patients.
- In two cases, patients required treatment and discontinued Qalsody, while the remaining four saw symptom resolution without discontinuation.
- Papilledema and elevated intracranial pressure have been reported in four patients, all of whom received standard care treatment with symptom resolution and no Qalsody discontinuations.
- Aseptic meningitis, chemical meningitis, or drug-induced aseptic meningitis occurred in one patient, leading to Qalsody discontinuation.
- Another patient experienced aseptic meningitis but continued treatment. Cases of increased CSF white blood cell and protein levels have also been reported.
- If symptoms indicative of these conditions develop, diagnostic and standard care treatment is warranted, which may require interrupting or discontinuing Qalsody.
- Regularly observe for symptoms and, if present, follow standard care procedures for diagnostic workup and treatment.
Common Qalsody Side Effects:
- Pain
- Fatigue
- Arthralgia
- Increased cerebrospinal fluid white blood cell
- Myalgia
- Headache
- Stiff neck
- Fever
- Blurred vision
- Change in vision
- Nausea
- Vomiting
- Confusion
Use in Specific Population
- The developmental risks of using Qalsody in pregnant women remain inadequately assessed, with no substantial data available to determine potential drug-related birth defects, miscarriage, or other maternal or fetal complications.
- The general population carries a background risk of 2 to 4% for major birth defects and 15 to 20% for miscarriages in recognized pregnancies in the U.S., but the specific background risks in the indicated population are undetermined.
- Animal studies involving subcutaneous administration of Tofersen to pregnant mice and rabbits during organogenesis revealed no adverse effects on embryo fetal development. However, there are no data available on Tofersen’s presence in human milk or its effects on breastfed infants and milk production.
- The safety and efficacy of Qalsody have not been established in pediatric patients, and while elderly patients were included in clinical studies, there were no notable differences in safety or effectiveness compared to younger patients. Age-based dosage adjustments are not indicated for Qalsody administration.
Storage and Handling
- Qalsody should be stored refrigerated at 2°C to 8°C (36°F to 46°F) within the original carton to shield it from light.
- Freezing should be avoided. In cases where refrigeration is unavailable, Qalsody can be kept in its original carton, protected from light, at or below 30°C (86°F) for up to 14 days.
- If removed from the original carton, unopened vials can be taken in and out of the refrigerator as needed for a maximum of 6 hours per day at or below 30°C (86°F), not exceeding 6 days (36 hours) in total.
Sansfro employs a four-step protocol for medication procurement to ensure patients can obtain their required prescription medications:
- Medication Inquiry: The Sansfro Named Access Programme Support team responds within 24 hours of receiving a patient’s inquiry to offer assistance.
- Verification Process: Sansfro verifies medication availability and approval, especially for medications that may be challenging to access in the patient’s home country. They review patient prescriptions and medical records for accuracy and compliance.
- Medication Procurement: Upon successful verification, Sansfro’s staff contacts their network of suppliers to promptly acquire the necessary medication while keeping a close eye on order processing to ensure reasonable costs.
- Safe Delivery: Following acceptance of the final quote, Sansfro arranges secure delivery of the medication. Their team of logistics specialists assists with shipment tracking and strictly adheres to standard operating procedures for the safe and authorized distribution of medications.
To initiate the medication import process, the Sansfro team will request the following documentation from patients:
- An authentic copy of their prescription.
- Documentation verifying their identity.
- Information about the prescribing physician.
- Current residential address.
Once these documents are received, the Sansfro team will proceed to submit the application for an import license. This license, once approved by the government, is a crucial prerequisite for acquiring the required medication.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What are the serious adverse reactions associated with Qalsody?
Serious adverse reactions reported in Qalsody patients include myelitis, radiculitis, papilledema, elevated intracranial pressure, and aseptic meningitis.
How should patients and healthcare providers manage these adverse reactions?
For patients experiencing symptoms consistent with these adverse reactions, diagnostic workup and treatment should be initiated according to the standard of care. Management may require interruption or discontinuation of Qalsody when necessary.
Is there a distinction between Qalsody and Tofersen?
No, Qalsody and Tofersen are essentially the same drug, both used to treat amyotrophic lateral sclerosis (ALS) in patients with a specific genetic mutation in the superoxide dismutase 1 (SOD1) gene. They refer to the identical therapeutic agent under different names.
Are there any known drug interactions with Qalsody?
No, there have been no clinical drug interaction studies conducted with Qalsody. In vitro studies have shown that Qalsody is not involved as a substrate, inhibitor, or inducer of major CYP enzymes or transporters.
How does Tofersen injection affect fertility and reproductive function?
In a study evaluating effects on fertility and reproductive function, Tofersen injection was administered to male and female mice prior to and during mating. Adverse effects were observed on male reproductive organs at the highest dose tested, but functional endpoints were unaffected. Plasma exposure at the no-effect dose was approximately 2 times that in humans at the recommended dose.
What is Qalsody price in India?
Qalsody price in India is based on specific product requirements. For additional information, please get in touch with our Patient Support Team at (+91) 93157 05373 or through email at help@sansfro.com.
How to buy Qalsody Online?
Contact any genuine Pharmaceutical Supplier who can help in importing the medicine after completing the necessary paperwork, as it may not be available everywhere.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.