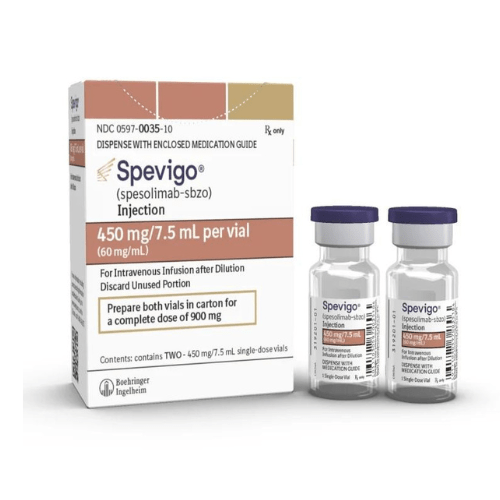
About Spesolimab-sbzo
- Spevigo received Food and Drug Administration (FDA) approval on September 1, 2022. Spevigo is an interleukin-36 receptor antagonist that belongs to the family called interleukin inhibitors.
- Spevigo, is specifically indicated for the treatment of generalized pustular psoriasis flares in adults. This medication is designed to address the symptomatic episodes associated with this skin condition, providing targeted relief for individuals experiencing flares of generalized pustular psoriasis.
- Spesolimab-sbzo, is a humanized monoclonal IgG1 antibody against human IL-36R, manufactured in Chinese hamster ovary (CHO) cells through recombinant DNA technology.
- Spevigo (spesolimab-sbzo) injection, with a molecular weight of about 146 kDa, is a sterile, preservative-free solution in a single-dose vial for intravenous infusion.
- Spesolimab-sbzo, a humanized monoclonal antibody, inhibits interleukin-36 (IL-36) signaling by binding specifically to the IL36R. This prevents the activation of IL36R by its ligands (IL-36 α, β, and γ), leading to the interruption of pro-inflammatory and pro-fibrotic pathways.
- While the precise connection between reduced IL36R activity and the treatment of generalized pustular psoriasis flares is not fully elucidated, Spesolimab-sbzo’s interference with IL-36 signaling is central to its mechanism of action.
- Pustular psoriasis flares are sudden and severe outbreaks of pustular psoriasis, a rare variation of psoriasis characterized by noninfectious, neutrophil-containing pustules on an erythematous background.
- Pustular psoriasis flares often occur with systemic symptoms, such as fever, chills, malaise, anorexia, nausea, severe pain, and itchiness.
- Generalized pustular psoriasis (GPP) is a severe form of pustular psoriasis that can cover large areas of the body and typically presents with fever, shivers, intense itching, a rapid pulse, fatigue, headache, nausea, muscle weakness, and joint pain.
- Obtaining advice from a healthcare professional is vital for precise diagnosis and proper management.
Strength:
Spevigo is available as an injection of 450 mg/7.5 mL (60 mg/mL) solution in a single-dose vial.
Recommended Dosage:
Spevigo requires dilution before administration. Visual inspection of the parenteral drug product is essential for particulate matter and discoloration assessment, and the solution should be discarded if cloudy, discolored, or contains large or colored particulates.
For preparation, adhere to aseptic techniques, withdraw and discard 15 mL from a 100 mL container of sterile 0.9% Sodium Chloride Injection, slowly replace it with 15 mL of Spevigo (contents from two vials of 450 mg/7.5 mL), and mix gently before use.
- Administer the diluted Spevigo solution as a single 900 mg dose through intravenous infusion over 90 minutes.
- In case of persistent flare symptoms, an additional 900 mg dose may be administered one week after the initial dose.
Important:
Do not combine Spevigo with other medicinal products. If the infusion is slowed or temporarily halted, ensure that the total infusion time, including any pause, does not exceed 180 minutes. For administration, a pre-existing intravenous line can be used, and it must be flushed with a sterile 0.9% Sodium Chloride Injection before and after the infusion. There should be no simultaneous administration of any other infusions through the same intravenous access.
Before initiating Spevigo injection, engage in a thorough discussion with your healthcare professional regarding your medical history and existing medications. Cease smoking, avoid alcohol consumption, and promptly communicate any new symptoms encountered while on Spevigo. Furnish your healthcare provider with a comprehensive list of all medications you are currently taking, encompassing prescriptions, over-the-counter drugs, vitamins, and herbal supplements. Maintain a record for reference and share it with your healthcare provider and pharmacist when introducing a new medication.
Warnings & Precautions
- Spevigo is contraindicated in patients with severe or life-threatening hypersensitivity to spesolimab-sbzo or any excipients in Spevigo, with reported reactions including drug reaction with eosinophilia and systemic symptoms (DRESS).
- The use of Spevigo may increase the risk of infections, as demonstrated in the Effisayil-1 trial, where 14% of treated subjects reported infections compared to 6% in the placebo group.
- In patients with chronic or recurrent infections, careful consideration of the risks and expected benefits is necessary before prescribing Spevigo.
- It is not recommended for patients with clinically important active infections. Tuberculosis (TB) evaluation is crucial before initiating Spevigo, and anti-TB therapy should be considered for those with latent TB or a history of TB.
- Hypersensitivity reactions, including anaphylaxis and DRESS, may occur, requiring immediate discontinuation of Spevigo and appropriate treatment.
- Infusion-related reactions should be addressed with medical therapy, and vaccinations with live vaccines are to be avoided in Spevigo-treated patients, with no specific studies conducted in this population for recently received live viral or bacterial vaccines.
Common Spevigo Side Effects:
- Feeling tired or weak
- Fatigue
- Nausea and vomiting
- Headache
- Itching or itchy bumps
- A collection of blood under the skin (bruising)
- Urinary tract infection
- Diarrhea
- Earache
- Ear drainage
- Itching ears
- Lack or loss of strength
- Change in hearing
Use in Specific Population
- Before commencing Spevigo treatment, it is essential to communicate any pregnancy plans or current pregnancy status to your doctor due to limited and insufficient data on Spevigo use in pregnant women, making it challenging to determine associated risks of adverse pregnancy outcomes.
- If breastfeeding or planning to breastfeed, notify your doctor, as the passage of Spevigo into breast milk is uncertain, with no available data. Discuss the most suitable feeding approach for your baby while on Spevigo.
- The safety and effectiveness of Spevigo in pediatric patients remain unestablished, and clinical studies lacked sufficient subjects aged 65 and over to ascertain potential differences in response compared to younger adults.
- Always consult your doctor, openly addressing any concerns, to ensure a safe and well-informed treatment plan.
Storage and Handling
- It is imperative to refrigerate Spevigo and store it at temperatures between 2°C to 8°C (36°F to 46°F) within the original carton to shield it from light.
- Avoid freezing. Unopened Spevigo vials can be stored at room temperature (20°C to 25°C or 68°F to 77°F) for up to 24 hours before use, provided they remain in the original carton to protect them from light.
- Keep the medicine away from pets and children.
Sansfro has developed a streamlined four-step process to facilitate the prompt acquisition of necessary prescription medications for patients:
- Medication Inquiry: When patients have queries about a specific medication, Sansfro’s Named Access Programme Support team responds within 24 hours.
- Verification Process: Sansfro meticulously reviews prescriptions and medical records to ensure accuracy and adherence, conducting assessments of medication availability and seeking necessary approvals.
- Procurement of Medication: Following the completion of the verification process, Sansfro’s team promptly contacts their network of suppliers to efficiently secure the required medication.
- Safe Delivery: Upon reaching an agreement on the price, Sansfro establishes a secure medication delivery system. Their logistics team ensures strict adherence to standard operating procedures for a safe and lawful distribution process.
To facilitate the importation of medication, patients are required to provide the following documentation:
- A valid copy of the prescription.
- Documentation confirming your identity.
- Details of the primary healthcare provider.
- Proof of current residence.
Once all necessary documents are submitted, the Sansfro team initiates the application process for import licenses. Upon government approval, this license becomes an essential requirement, facilitating the smoother procurement of the required medication.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What is Spevigo used for?
Spevigo is an interleukin-36 receptor antagonist indicated for the treatment of generalized pustular psoriasis (GPP) flares in adults.
How is Spevigo administered?
Spevigo is administered as an intravenous infusion, typically as a single 900 mg dose over 90 minutes.
Is Spevigo safe for use in pregnancy?
Limited data are available on the use of Spevigo in pregnant women, and its safety during pregnancy is uncertain. Consult your doctor if you are pregnant or planning to become pregnant.
Can I breastfeed while using Spevigo?
It is uncertain whether Spevigo passes into breast milk. Consult your doctor before breastfeeding while undergoing treatment.
What are the common side effects of Spevigo?
Common side effects include nausea, diarrhea, vomiting, fatigue, musculoskeletal pain, hepatotoxicity, renal impairment, edema, dyspnea, and decreased appetite. If you experience any side effects, it is recommended to inform your healthcare professional immediately.
How should Spevigo be stored?
Spevigo should be refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect it from light. Do not freeze. Unopened vials may be stored at room temperature (20°C to 25°C or 68°F to 77°F) for up to 24 hours in the original carton.
Can Spevigo be used with other medications?
Do not mix Spevigo with other medicinal products. Consult your healthcare provider for guidance on potential drug interactions and inform them about any other medications you are taking to avoid interactions.
What precautions should be taken with Spevigo?
Precautions include monitoring for hypersensitivity reactions, infections, tuberculosis risk, and avoiding live vaccines during Spevigo treatment.
What is the Spevigo cost in India?
Spevigo price in India is determined by specific product(strength & Quantity) requirements. For detailed pricing information, please reach out to our Patient Support Team at (+91) 9315705373 or via email at help@sansfro.com.
How can I buy Spevigo online?
If you are considering buying Spevigo online, now available in both the US and Europe, we recommend contacting the Sansfro Health team or other reputable organizations specializing in importing medications from these regions. Ensure a secure and reliable process by consulting with experienced professionals in the field.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.