About Ultomiris
- Ultomiris is an monoclonal antibody which belongs to the family called complement Inhibitors.
- Ultomiris is used for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) in adult and pediatric patients who are one month of age and older.
- PNH is a rare blood disorder that causes red blood cells to break down prematurely. aHUS is a rare kidney disorder that can lead to kidney failure.
- Ultomiris is works by blocking a protein called C5. C5 is a part of the immune system that helps to fight infection. However, in people with PNH and aHUS, C5 can also attack healthy blood cells and kidneys. By blocking C5, Ultomiris can help to prevent the destruction of blood cells and kidneys in people with PNH and aHUS.
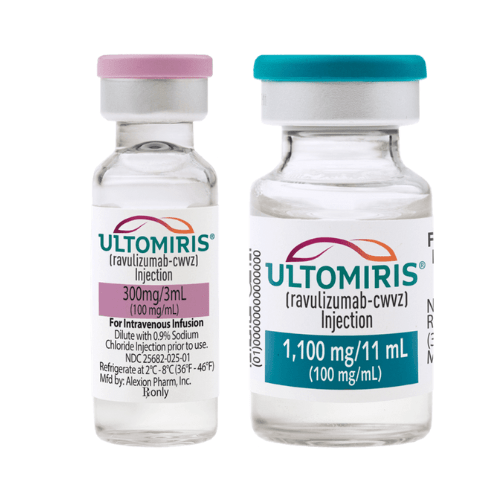
- Ultomiris injection at a concentration of 100mg/mL is a sterile, transparent liquid with a clear to slightly yellowish hue. It is free from preservatives and is intended for intravenous administration.
- It is necessary to deliver this medication on a regular basis by injections or infusions under the guidance of a medical practitioner. It’s crucial to speak with a healthcare professional for advice and more detailed information about the procedure.
Strength:
Injection: IV infusion of 300mg/30mL (10mg/mL); 300mg/3mL (100mg/mL); 1,100mg/11mL (100mg/mL) in a single-dose vial.
Recommended Dosage:
The drug is administered by a health care professional once a week directly into the bloodstream through intravenous. Follow current ACIP guidelines for meningococcal disease vaccination to minimize the risk of severe infection. If Ultomiris needs immediate initiation and vaccines were given less than 2 weeks before starting Ultomiris treatment, provide 2 weeks of antibacterial prophylaxis.
- For adult and pediatric patients aged one month or older, weighing 5kg or more with PNH, aHUS, or adult patients with gMG weighing 40kg or more, the dosing includes a loading dose followed by maintenance doses via intravenous infusion.
- The dosing regimen depends on the patient’s body weight. Maintenance doses should commence two weeks after the loading dose and occur every 4 or 8 weeks, depending on body weight.
- While minor variations are permitted within 7 days of the scheduled infusion day (excluding the first maintenance dose of Ultomiris), subsequent doses should adhere to the original schedule.
- After each infusion, you should be monitored for at least 1 hour for infusion reactions.
Important:
To prepare Ultomiris, visually inspect vials for any particulate matter or precipitation and withdraw the required volume based on the patient’s weight and dose. Dilute it in an infusion bag using 0.9% Sodium Chloride Injection, USP to achieve a final concentration of 50mg/mL (3mL and 11mL vials) or 5mg/mL (30mL vial). Gently mix without shaking, protect from light, and don’t freeze. Administer immediately or store refrigerated for up to 24 hours (considering infusion time). For administration, use a 0.2 or 0.22 micron filter, ensure room temperature, and visually inspect for particulates and discoloration prior to use.
Do not discontinue treatment without your physician’s guidance. If you discontinue Ultomiris treatment for PNH, your healthcare provider will require careful monitoring for a minimum of 16 weeks afterward. Ceasing Ultomiris could lead to the destruction of red blood cells as a result of PNH. For any questions or concerns, consult your healthcare provider and provide a complete list of all medications, including OTC drugs and herbal supplements, that you are currently using.
Warnings & Precautions
- Ultomiris is contraindicated in patients with unresolved Neisseria meningitidis infection and in patients who are not currently vaccinated against Neisseria meningitidis, unless the risks of delaying Ultomiris treatment outweigh the risks of developing a meningococcal infection.
- Exercise caution when considering Ultomiris treatment for patients with any other systemic infection.
- Close monitoring of patients during infusion is essential. In the event of reactions, promptly interrupt the infusion and implement appropriate supportive measures.
- The use of Ultomiris in pregnant women has not been studied, and its potential risks to the fetus are unknown.
- Animal studies with a similar antibody to Ultomiris indicated an increased risk of developmental abnormalities and fetal mortality at doses higher than the recommended human dose.
- Pregnant individuals with PNH and aHUS are at risk of adverse maternal and fetal outcomes, including thrombotic events, infections, bleeding, miscarriages, and maternal mortality.
- It is unknown whether Ultomiris is present in human milk or its effects on breastfed infants and milk production.
- Since many drugs and immunoglobulins are excreted in human milk and due to potential serious adverse reactions in nursing infants, breastfeeding should be discontinued during Ultomiris treatment and for 8 months following the final dose.
- Please consult your healthcare provider for further guidance regarding pregnancy and lactation considerations.
Common Ultomiris Side Effects:
- Upper respiratory tract infection
- Headache
- Diarrhea
- Nausea
- Vomiting
- Hypertension
- Pyrexia
- Infusion-related reactions
- Back pain
- Abdominal pain
- Urinary tract infection
- Fatigue
- Dizziness
- Muscle pain
- Arthralgia (joint pain)
- Neisseria meningitidis infection (meningitis)
- Streptococcus pneumoniae infection (pneumococcal infection)
- Haemophilus influenzae type b infection (Hib infection)
- Other serious infections
Use in Specific Population
- Ultomiris is proven effective for treating PNH in pediatric patients aged one month and older, supported by data from adult trials and additional information in pediatric patients aged 9 to 17.
- Its use in pediatric patients aged less than 9 years and body weight <30kg is based on extrapolated data from aHUS and PNH studies.
- For aHUS, Ultomiris has been shown to work in pediatric patients aged one month and older, with data from adults and pediatric patients aged 10 months to <17 years. However, there is no established use of Ultomiris for pediatric patients with gMG.
- Limited data are available for patients aged 65 and over, no significant differences in responses have been identified compared to younger patients.
Storage and Handling
- Store Ultomiris vials refrigerated at 2°C – 8°C (36°F – 46°F) in the original carton.
- Protect the medicine from light. Do not freeze. Do not shake.
- Remember that Ultomiris is a sterile product, so do not use it if the vial is damaged or the seal is broken.
- Always employ aseptic techniques and discard any unused portions. Never reuse the vial or infusion set to maintain safety and effectiveness.
For the medicine procurement, we follow a simple four-step process.
- Enquiry about the medicine: When you request information about the medication you require, we will handle your data. Our Named Access Program Support team will contact you within 24 hours to assist.
- Verification Process: In our mission to help patients access medications that may not be approved or readily accessible in their home countries, Sansfro ensures the verification of medicine availability and approval. Additionally, we thoroughly review the patient’s prescription and medical information for accuracy and compliance.
- Sourcing the Medicine: Upon successfully completing the verification process, our team will initiate contact with our network of suppliers to source the required medication for you. Subsequently, our team works diligently to obtain the most favorable quotes for your medicines and oversees the processing of your order.
- Safe Delivery: We will coordinate the secure delivery of your consignment upon approval of the final quote. Our team of logistics specialists is available to provide consignment tracking assistance. Acknowledging that the Named Access Program industry is susceptible to unauthorized channels is essential. To uphold the safe and legal provision of medications, we rigorously adhere to Standard Operating Procedures.
For the importation of medication, we will require the following documents from the patient:
- An authentic prescription copy.
- A proof of identity document.
- Information about the healthcare provider in charge.
- A current residential address.
Once all these documents are provided, the Sansfro team will begin the import license application process. This license is a crucial requirement to facilitate the procurement of the necessary medication upon receiving government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
Can Ravulizumab-cwvz be used to treat patients with Shiga toxin E.coli-related hemolytic uremic syndrome (STEC-HUS)?
No, Ravulizumab-cwvz cannot be used to treat patients with Shiga toxin E.coli-related hemolytic uremic syndrome (STEC-HUS). It is not indicated for this condition.
How should vaccination and antibiotic prophylaxis be managed with Ravulizumab-cwvz treatment?
Patients receiving Ravulizumab-cwvz should be vaccinated for meningococcal disease as per current Advisory Committee on Immunization Practices (ACIP) guidelines to reduce the risk of serious infection. Antibacterial drug prophylaxis should be provided for 2 weeks if Ravulizumab-cwvz must be initiated immediately and vaccines are administered less than 2 weeks before starting the medicine.
What should I do if I start a new medicine while taking Ravulizumab-cwvz?
If you begin a new medication while already using Ravulizumab-cwvz, make sure to inform your doctor and pharmacist promptly. They can evaluate potential interactions and advise you on the best course of action.
Why is it important to tell my doctor about all the medicines I take?
It’s essential to inform your doctor about all the medicines you take, including prescription and over-the-counter drugs, vitamins, and herbal supplements. This helps your healthcare provider assess potential interactions and side effects when you’re prescribed Ravulizumab-cwvz or any other medication.
What is the price of Ravulizumab-cwvz in India?
Ravulizumab-cwvz price in India depends on the product requirement. Request more details by contacting our Patient Support Team at (+91) 93157 05373 or help@sansfro.com.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.