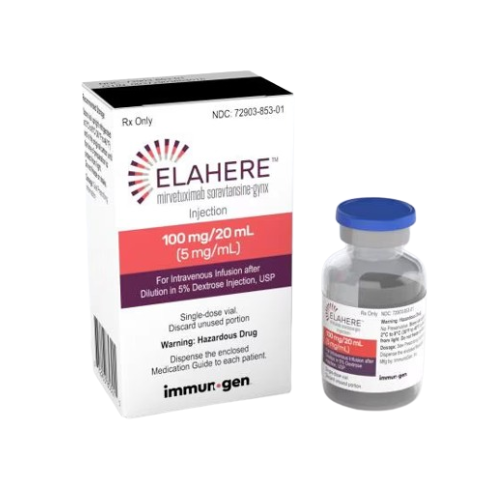
About Elahere
- Elahere received approval from the US FDA administration on November 14, 2022
- Elahere is an antineoplastic,which belongs to the class of miscellaneous antineoplastics.
- Elahere is a treatment designed for adult patients with folate receptor alpha (FRα)-positive platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.
- This antibody and microtubule inhibitor conjugate is indicated for individuals who have undergone one to three prior systemic treatment regimens.
- Your physician will conduct tests to ensure that Elahere is the appropriate treatment for you. The safety and effectiveness of Elahere in children are not known.
Strength
Injection: 100 mg/20 mL (5 mg/mL) in a single-dose vial.
Recommended Dosage
Before administering parenteral drug products, visually inspect for particulate matter and discoloration whenever the solution and container permit. Elahere is a clear to slightly opalescent, colorless solution. Ensure not to freeze the prepared infusion solution.
- For Elahere injection, the suggested dose is 6 mg per kilogram of your adjusted ideal body weight.
- This is given through an intravenous infusion every 3 weeks, following a 21-day cycle.
- This continues until either your disease progresses or there are side effects that are too severe to tolerate.
- Before the procedure, use a corticosteroid, antihistamine, and antipyretic as premedication. Additionally, premedicate with an antiemetic, ophthalmic topical steroids, and lubricating eye drops.
Important
Before commencing the new medication, it is crucial to notify your doctor if you are presently taking any medications. Patients are strongly encouraged to share comprehensive information about all concurrent medications, including prescription drugs, over-the-counter medications, vitamins, and herbal products, with their healthcare providers. This open communication ensures a thorough understanding of the patient’s medication profile and aids in safe and effective treatment planning.
Warnings & Precautions
- Elahere may lead to severe ocular toxicities, causing visual impairment, keratopathy, dry eye, photophobia, eye pain, and uveitis.
- Before initiating Elahere and every other cycle for the first 8 cycles, as well as when clinically indicated, conduct an ophthalmic exam, including visual acuity and slit-lamp examination.
- Prophylactic artificial tears and ophthalmic topical steroids should be administered. In case of ocular toxicities, withhold Elahere until improvement and resume at the same or reduced dose.
- Discontinue Elahere for Grade 4 ocular toxicities. For pneumonitis, withhold Elahere for persistent or recurrent Grade 2 cases and consider dose reduction. Permanently discontinue Elahere for Grade 3 or 4 pneumonitis.
- Monitor patients for new or worsening peripheral neuropathy, and, based on severity, withhold dosage, reduce dose, or permanently discontinue Elahere. It is crucial to promptly inform your physician if you experience any symptoms related to these conditions.
Common Elahere Side Effects:
- Vision impairment
- Fatigue
- Increased aspartate aminotransferase
- Nausea
- Increased alanine aminotransferase
- Keratopathy
- Abdominal pain
- Decreased lymphocytes
- Peripheral neuropathy
- Diarrhea
- Decreased albumin
- Constipation
- Increased alkaline phosphatase
- Dry eye
- Decreased magnesium
- Decreased leukocytes
- Decreased neutrophils
- Decreased hemoglobin
Use in Specific Population
- Elahere poses a risk of embryo-fetal harm due to the presence of the genotoxic compound DM4, affecting actively dividing cells. Hence, it is essential to inform your doctor if you are pregnant, planning pregnancy, or suspect pregnancy before starting treatment.
- Breastfeeding is not advised during Elahere treatment and for one month after the last dose due to potential serious adverse reactions in the child.
- Females of reproductive age should employ effective contraception during Elahere treatment and for seven months post-treatment.
- The safety and efficacy of Elahere are not established in pediatric patients.
- In patients aged 65 and older, no clinically significant differences in efficacy or safety were noted compared to younger patients.
- No dosage adjustment is recommended for mild to moderate renal impairment (CLcr 30 to 90 mL/min), but Elahere use is discouraged in patients with moderate or severe hepatic impairment.
- For more information on fertility geriatric, and pediatric use, consult your healthcare provider.
Storage and Handling
- Store within the temperature range of 2°C to 8°C (36°F to 46°F).
- Protect Elahere from light and moisture.
- Do not freeze.
- Do not store Elahere where it may be exposed to heat or humidity.
- Keep Elahere out of the reach of children and pets.
Sansfro has developed a comprehensive four-step process to expedite patients’ acquisition of necessary prescription medications:
- Medication Inquiry: When patients ask questions regarding a specific medication, Sansfro’s Named Access Programme Support team responds to them within 24 hours.
- Verification Process: Sansfro meticulously reviews prescriptions and medical records to ensure accuracy and adherence. They also assess medication availability and seek necessary approvals.
- Procurement of Medication: Once the verification is complete, Sansfro’s team contacts their network of suppliers promptly to efficiently secure the required medication.
- Safe Delivery: After agreeing on the price, Sansfro establishes a secure medication delivery system. Their logistics team ensures the adherence to standard operating procedures for a safe and lawful distribution process.
We will need the patient to provide the following paperwork in order to import medication:
- A genuine copy of the prescription.
- A document proving your identity.
- Details about the lead healthcare provider.
- A residence that is currently in use.
After receiving all of these documents, the Sansfro team will start the application process for import licences. After government approval, this licence is an essential requirement to make it easier to purchase the required medication.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
How often do I take Elahere?
Elahere is typically given every 3 weeks, and the amount is determined based on your adjusted ideal body weight. This is done through an intravenous infusion, meaning it goes directly into your vein. You’ll continue with this treatment until either your condition gets worse or if there are side effects that are too severe. It’s important to follow your healthcare provider’s guidance on this.
What is Elahere used for?
Elahere is indicated for the treatment of adult patients diagnosed with folate receptor-alpha (FRα) positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer. This applies to cases where the patient has undergone one to three prior systemic treatment regimens, and Elahere serves as a therapeutic option for those facing challenges in managing their condition.
Can Elahere be used in patients with liver impairment?
Elahere is not recommended for use in patients with moderate or severe liver impairment, particularly those with a total bilirubin level exceeding 1.5 times the upper limit of normal (ULN). However, for patients with mild liver impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin > 1 to 1.5 times ULN with any AST elevation), no dosage adjustment is necessary.
How do Elahere and Mirvetuximab soravtansine differ?
Elahere and Mirvetuximab soravtansine are synonymous medications. Elahere represents the branded version, whereas Mirvetuximab soravtansine is the generic name or active pharmaceutical ingredient (API) of the drug. Essentially, they are identical medications, differing only in nomenclature.
How to buy Elahere Online?
Procuring Elahere online has become increasingly convenient. To get this medication, which is presently available in the US and Europe, consider reaching out to the SANSFRO team or other specialized organizations with expertise in importing medications from these regions.
What is the Elahere price in India?
Elahere price in India is determined by the specific product requirements. For further information, please contact our Patient Support Team at (91) 93157 05373 or send an email to help@sansfro.com.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.