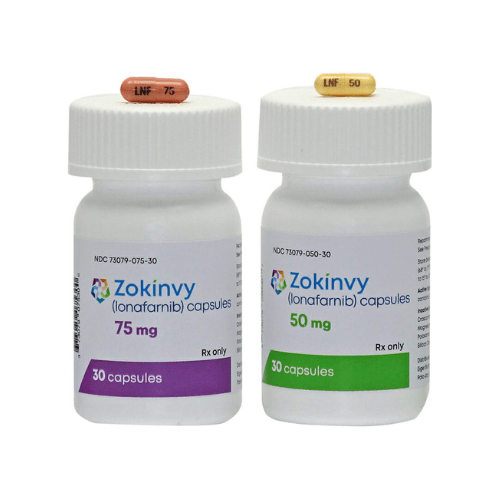
About Lonafarnib
- Lonafarnib is a farnesyltransferase inhibitor prescribed for patients aged 12 months and older, with a body surface area of 0.39m2 and above.
- Lonafarnib belongs to the class miscellaneous metabolic agents which serves a dual purpose, aiming to reduce the risk of mortality associated with Hutchinson-Gilford Progeria Syndrome and providing treatment for processing-deficient Progeroid Laminopathies.
- The latter encompasses cases with a heterozygous LMNA (lamin A/C gene) mutation featuring progerin-like protein buildup, as well as those with homozygous or compound heterozygous ZMPSTE24 mutations.
- The selection of patients for Lonafarnib integration into their treatment plan is determined by their medical history, diagnosis, and the guidance of their healthcare provider.
Strength:
Lonafarnib hard capsules are delivered in a strength of 50mg and 75mg, respectively, for oral consumption.
Recommended Dosage:
For patients able to swallow capsules, take Lonafarnib capsules whole with water; do not chew. If unable to swallow, mix the capsule contents with Ora Blend SF® or OraPlus®. Alternatively, for those unable to use these options, mix with orange juice or applesauce (avoid grapefruit or Seville oranges).
Prepare each mixture fresh for each dose and consume within about 10 minutes. To prepare in Ora Blend SF, Ora-Plus, or orange juice, empty capsule contents into 5 mL to 10 mL of liquid, mix, and consume the entire serving. Mix capsule contents with 1 to 2 teaspoonfuls for applesauce, stir well, and consume the entire serving.
- The initial Lonafarnib dosage for patients with a BSA (Body Surface Area) of 0.39m² and above is 115mg/m², taken twice daily with morning and evening meals to minimize the risk of gastrointestinal side effects.
- Following four months of treatment, the dosage should be increased to 150mg/m², also taken twice daily with meals.
- Dosages should be rounded to the nearest 25mg increment. If a dose is missed, take it with food as soon as possible, up to 8 hours before the next scheduled dose.
- If less than 8 hours remain, skip the missed dose and resume at the next scheduled time.
Important:
Ensure you don’t miss any doses of Lonafarnib. If you happen to miss a dose, take it as soon as possible. However, if your next dose is approaching, skip the missed one and resume your regular schedule never take two doses together. Keep your doctor informed about all medications, including over-the-counter drugs, herbal supplements, and vitamins, as some may interact with Lonafarnib, potentially leading to severe side effects. If you have questions or concerns about taking this medicine , discuss them with your doctor. It’s crucial to adhere to these instructions carefully to maximize the benefits of the medicine while minimizing the risk of side effects.
Warnings & Precautions
- Lonafarnib can cause serious birth defects if taken during pregnancy. Pregnant women or those planning to become pregnant should avoid Lonafarnib.
- Lonafarnib may lead to liver damage, which can be life-threatening. Regular liver function monitoring is essential for patients using Lonafarnib.
- Lonafarnib can reduce blood cell counts, increasing the risk of infection, bleeding, and anemia. Patients should undergo regular blood count monitoring.
- Lonafarnib may trigger hypersensitivity reactions, including anaphylaxis. Patients should be monitored for such reactions during and after administration.
- Caution is advised when using Lonafarnib in patients with liver issues. Close monitoring for signs of hepatotoxicity is necessary.
- Patients with kidney problems should use Lonafarnib cautiously and be closely monitored for signs of myelosuppression.
- Lonafarnib can interact with other medications, including over-the-counter drugs, supplements, and vitamins. Patients should inform their doctor of all medications they are taking before starting Lonafarnib.
- Lonafarnib can cause serious birth defects, making it unsuitable for pregnant women. Immediate medical contact is necessary if pregnancy occurs during Lonafarnib use.
- It is unknown if Lonafarnib passes into breast milk, and because it may harm nursing infants, breastfeeding should be avoided by women taking Lonafarnib
Common Lonafarnib Side Effects:
- Serious liver damage
- Myelosuppression (a decrease in the number of blood cells)
- Hypersensitivity reactions, including anaphylaxis
- Dizziness
- Fatigue
- Infections
- Constipation
- Increased blood pressure
- Decreased weight
- Electrolyte abnormalities
- Hair loss
- Nail changes
- Skin discoloration
Use in Specific Population
Lonafarnib has not been studied in patients aged 65 and above. It is indicated for use in patients aged one year and older with Hutchinson-Gilford Progeria Syndrome (HGPS) and processing-deficient Progeroid Laminopathies (PL). Caution is advised when using the medicine in patients with liver or kidney impairment, and close monitoring for signs of liver damage (hepatotoxicity) and blood cell count reduction (myelosuppression) is necessary.
Storage and Handling
Before handling Lonafarnib capsules, ensure your hands are clean by washing them with soap and water. Avoid touching the capsules with wet hands. If you accidentally touch a capsule with wet hands, make sure your hands are completely dry before handling the capsule again.
- Store Lonafarnib capsules at room temperature (68°F to 77°F or 20°C to 25°C).
- Protect Lonafarnib from light and moisture.
- Do not store Lonafarnib in the bathroom or in other areas where it may be exposed to heat or humidity.
Keep Lonafarnib out of the reach of children and pets
For the medicine procurement, we follow a simple four-step process.
- Enquiry about the medicine: When you request information about the medication you require, we will handle your data. Our Named Access Program Support team will contact you within 24 hours to assist.
- Verification Process: In our mission to help patients access medications that may not be approved or readily accessible in their home countries, Sansfro ensures the verification of medicine availability and approval. Additionally, we thoroughly review the patient’s prescription and medical information for accuracy and compliance.
- Sourcing the Medicine: Upon successfully completing the verification process, our team will initiate contact with our network of suppliers to source the required medication for you. Subsequently, our team works diligently to obtain the most favorable quotes for your medicines and oversees the processing of your order.
- Safe Delivery: We will coordinate the secure delivery of your consignment upon approval of the final quote. Our team of logistics specialists is available to provide consignment tracking assistance. Acknowledging that the Named Access Program industry is susceptible to unauthorized channels is essential. To uphold the safe and legal provision of medications, we rigorously adhere to Standard Operating Procedures.
For the importation of medication, we will require the following documents from the patient:
- An authentic prescription copy.
- A proof of identity document.
- Information about the healthcare provider in charge.
- A current residential address.
Once all these documents are provided, the Sansfro team will begin the import license application process. This license is a crucial requirement to facilitate the procurement of the necessary medication upon receiving government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What is the use of Zokinvy?
Zokinvy (lonafarnib) is a medication used to treat two uncommon genetic conditions associated with premature aging:
- Hutchinson-Gilford progeria syndrome (HGPS): This severe childhood illness manifests with accelerated aging symptoms, including stunted growth, wrinkled skin, hair loss, and cardiovascular issues. Zokinvy has demonstrated efficacy in reducing the risk of death in HGPS patients and improving certain symptoms.
- Processing-deficient progeroid laminopathies (PDPLs): Representing a group of rarer conditions sharing similarities with HGPS, PDPLs result from genetic mutations affecting the processing of the protein lamin A. Zokinvy aids in symptom management and has the potential to slow disease progression in select PDPL cases.
How to buy Zokinvy online?
For those contemplating the online acquisition of Zokinvy, now available in both the US and Europe, we recommend reaching out to the Sansfro Health team or other reputable organizations specializing in medication importation from these regions. Ensure a secure and reliable process by consulting with experienced professionals. Sansfro Health is a trusted and reputable company in this regard.
How does Zokinvy work?
Zokinvy works by blocking the activity of farnesyltransferase, an enzyme that is involved in the production of proteins that are essential for cell growth and division. By blocking the activity of farnesyltransferase, Zokinvy can help to slow the progression of HGPS and PL.
Who should not take Zokinvy?
Zokinvy should not be taken by people with severe liver or kidney disease, a history of allergic reactions to lonafarnib or other FTIs and if you are pregnant or breastfeeding. Before starting to consume the medicine, inform your doctor about existing medical conditions.
What foods and drinks should I avoid while taking Zokinvy?
You should avoid foods and drinks that contain grapefruit, cranberries, pomegranates, or Seville oranges (e.g., orange marmalade), otherwise known as sour or bitter oranges. Taking Zokinvy with food or drinks containing these fruits or fruit juices may increase adverse reactions associated with this medicine.
Is it okay to consume alcohol while taking Zokinvy?
Combining Zokinvy with alcohol is not advisable, as it raises the risk of liver damage. Alcohol is a toxic substance that can harm the liver as it undergoes liver processing, generating harmful chemicals that damage liver cells. Taking the medicine and alcohol concurrently intensifies the strain on the liver, resulting in greater production of these harmful chemicals and increased liver damage risk due to the dual impact of the medicine and alcohol on the liver.
What is the difference between Lonafarnib and Zokinvy capsules?
Lonafarnib and Zokinvy capsules are the same medication. Zokinvy is the brand name for lonafarnib, which is a generic drug. If you are considering taking lonafarnib or Zokinvy, please talk to your doctor to discuss the risks and benefits of these medications.
What is the price of Zokinvy in India?
Zokinvy price in India depends on the product requirement. Request more details by contacting our Patient Support Team at (+91) 93157 05373 or help@sansfro.com.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.