About Golodirsen
- Golodirsen is an antisense oligonucleotide belonging to the family called Morpholino antisense Oligomers.
- Golodirsen is used for the treatment of Duchenne Muscular Dystrophy (DMD) in patients with a verified mutation in the DMD gene that can be addressed through exon 53 skipping.
- Exon 53 skipping represents a form of gene therapy employing antisense oligonucleotides to obstruct the generation of dystrophin protein that lacks exon 53, permitting the body to produce a truncated yet functional dystrophin protein.
- The accelerated approval of this indication has been granted based on the observed increase in dystrophin production within skeletal muscle in patients treated with Golodirsen.
- To maintain this approval, it may be necessary to confirm and elucidate the clinical benefits through a subsequent confirmatory trial.
- This treatment is typically administered under the supervision of a healthcare professional and may require regular injections or infusions. It’s important to consult with a healthcare provider for more specific information and guidance regarding the process.
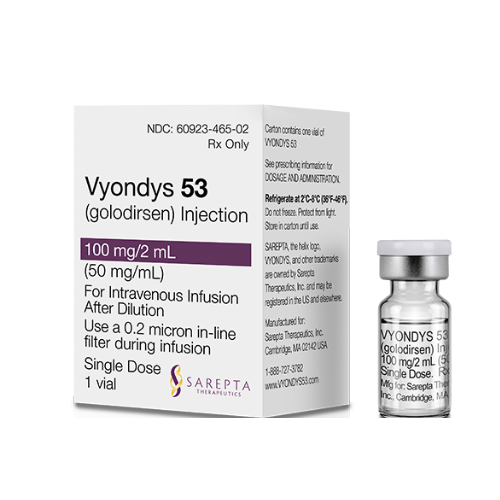
Strength:
Injection: IV infusion of 100mg/2mL (50mg/mL) in a single-dose vial.
Recommended Dosage:
The drug is administered by a health care professional once a week directly into the bloodstream through intravenous (a vein) or IV infusion. It takes approximately an hour for Golodirsen to be infused into the body. Before beginning the treatment, it is advisable to assess the levels of serum cystatin C, urine protein-to-creatinine ratio, and urine dipstick.
- A weekly dose of 30mg/kg of body weight is recommended for. As an intravenous infusion, it takes 35 to 60 minutes and is injected through an inline 0.2 micron filter.
- Patients may need to adjust the dosage of medication according to their response and side effects.
- The recommended dosage of Golodirsen for your individual needs should be discussed with a healthcare professional.
Important:
Discontinuing the treatment should only occur under the guidance of your physician. Importantly, this medication should not be considered a replacement for standard care. Individuals using Golodirsen should continue to receive all recommended treatments, such as physical therapy and occupational therapy. If you have any questions or concerns, please don’t hesitate to discuss them with your healthcare provider.
To ensure your safe and effective use of Golodirsen, it’s crucial to provide a comprehensive medical history, which includes any allergies, infections, or existing medical conditions. Make sure to disclose all medications you are currently taking, including over-the-counter drugs and herbal supplements.
Warnings & Precautions
- Golodirsen can trigger a severe allergic reaction known as hypersensitivity reactions. Patients must undergo close monitoring for anaphylactic signs and symptoms during and after each infusion and discontinuation is needed if such reactions occur.
- The hypersensitivity reaction includes, rash, pyrexia, pruritus, urticaria, dermatitis, and skin exfoliation have occurred in patients who were treated with Golodirsen.
- Golodirsen has been associated with kidney toxicity in animal studies, and although clinical studies didn’t show kidney toxicity.
- Limited clinical experience exists, and kidney issues have been observed with some antisense oligonucleotides. Therefore, kidney function should be monitored in patients using Golodirsen, especially in patients with pre-existing kidney disease.
- Golodirsen is not recommended for pregnant and breastfeeding women unless it is advised by a doctor. Because, the safety and efficacy is unknown in such population.
- Golodirsen has not been specifically studied in patients with liver impairment. However, patients with hepatic impairment may be at increased risk for developing hepatotoxicity.
- Golodirsen should be used with caution in patients taking other medications that may interact with the current medicine.
Common Golodirsen Side Effects:
- Headache
- Pyrexia
- Abdominal pain
- Nasopharyngitis
- Cough
- Vomiting
- Rash
- Nausea
- Pain at the injection site
Use in Specific Population
- Golodirsen has not been studied in pregnant or lactating women, so there is no way to know if it is safe or effective for these populations. If you are pregnant or breastfeeding, talk to your doctor about the risks and benefits of the medicine.
- Golodirsen can be given to children as young as 6 months old, but it hasn’t been studied in older adults. However, older people might be more prone to certain side effects, like reactions during the infusion and potential liver issues.
Storage and Handling
- Store Golodirsen at temperatures between 2°C to 8°C (36°F to 46°F) and ensure it does not freeze.
- Avoid shaking the vial and protect it from exposure to light.
- Remember that Golodirsen is a sterile product, so do not use it if the vial is damaged or the seal is broken.
- Always employ aseptic techniques and discard any unused portions. Never reuse the vial or infusion set to maintain safety and effectiveness.
For the medicine procurement, we follow a simple four-step process.
- Enquiry about the medicine: When you request information about the medication you require, we will handle your data. Our Named Access Program Support team will contact you within 24 hours to assist.
- Verification Process: In our mission to help patients access medications that may not be approved or readily accessible in their home countries, Sansfro ensures the verification of medicine availability and approval. Additionally, we thoroughly review the patient’s prescription and medical information for accuracy and compliance.
- Sourcing the Medicine: Upon successfully completing the verification process, our team will initiate contact with our network of suppliers to source the required medication for you. Subsequently, our team works diligently to obtain the most favorable quotes for your medicines and oversees the processing of your order.
- Safe Delivery: We will coordinate the secure delivery of your consignment upon approval of the final quote. Our team of logistics specialists is available to provide consignment tracking assistance. Acknowledging that the Named Access Program industry is susceptible to unauthorized channels is essential. To uphold the safe and legal provision of medications, we rigorously adhere to Standard Operating Procedures.
For the importation of medication, we will require the following documents from the patient:
- An authentic prescription copy.
- A proof of identity document.
- Information about the healthcare provider in charge.
- A current residential address.
Once all these documents are provided, the Sansfro team will begin the import license application process. This license is a crucial requirement to facilitate the procurement of the necessary medication upon receiving government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
Can kidney toxicity be a concern with Vyondys 53?
Based on animal data, Vyondys 53 may have the potential to cause kidney toxicity. Therefore, it is recommended to monitor kidney function in patients receiving Vyondys 53. It’s important to note that creatinine levels may not always provide an accurate measure of renal function in patients with Duchenne muscular dystrophy (DMD).
How to manage the side effects of Golodirsen?
Promptly report any side effects or concerns to your healthcare provider, who may recommend pre-medications, adjust the infusion rate, and conduct regular monitoring, including kidney function checks, to ensure the safe and effective use of Golodirsen .
Is there a correlation between exon skipping and dystrophin levels in response to Vyondys 53?
Yes, there is a positive correlation between the level of exon skipping and the increase in dystrophin protein expression observed in patients treated with Vyondys 53.
What is the structure of Golodirsen?
Golodirsen is classified as an antisense oligonucleotide, specifically a phosphorodiamidate morpholino oligomer (PMO). It consists of synthetic molecules with morpholino rings linked by uncharged phosphorodiamidate moieties.
What is Golodirsen and how it relates to Vyondys 53?
Golodirsen and Vyondys 53 are the same drug. Vyondys 53 is the brand name and Golodirsen is a generic name. They are both the same drug with the same mechanism of action and the same target population.
What is the price of Vyondys 53 in India?
Vyondys 53 price in India depends on the product requirement. Request more details by contacting our Patient Support Team at (+91) 93157 05373 or help@sansfro.com.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.