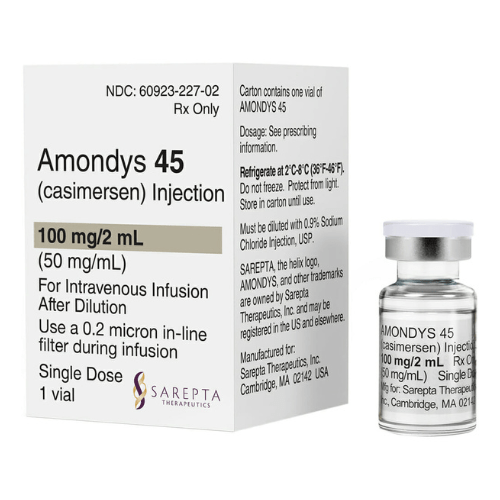
About Casimersen
- On February 25, 2021, the FDA approved Amondys 45, also known as casimersen.
- Amondys 45 contains casimersen, an antisense oligonucleotide derived from Sarepta’s phosphorodiamidate morpholino oligomer (PMO) platform.
- Amondys 45 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients with confirmed mutations of the DMD gene amenable to exon 45 skipping. This approval is based on observed increases in dystrophin production within skeletal muscle, which is a predictive marker of clinical benefit for DMD patients.
- Amondys 45 acts as an antisense oligonucleotide targeting specific mutations in the DMD gene, facilitating exon 45 skipping. This process restores the gene’s reading frame, leading to the synthesis of a truncated yet functional dystrophin protein. Dystrophin is crucial for muscle function, and its deficiency characterizes DMD. By promoting the production of a partially functional dystrophin protein, Amondys 45 aims to enhance muscle function and decelerate disease progression in DMD patients.
- Clinical trials of Amondys 45 demonstrated a statistically significant increase in dystrophin production within skeletal muscle, indicating potential clinical benefit for DMD patients. The drug exhibited promising results in augmenting dystrophin levels, which are essential for muscle health and function in individuals with DMD.
- Patients treated with Amondys 45 experienced notable improvements in dystrophin levels, suggesting potential clinical benefits for managing Duchenne muscular dystrophy.
Strength:
- Each single-dose vial contains 100 mg/2 mL of Casimersen.
Recommended Dosage:
Consider antiemetics before and during Amondys 45 treatment. Inspect drug products visually before administration. Reconstitute by shaking vial(s) with 40 mL of 5% Dextrose Injection, USP, then withdraw 80 mL from a refrigerated 250 mL infusion bag of 0.9% Sodium Chloride Injection, USP. Discard 80 mL and transfer the reconstituted solution to achieve a concentration of 0.1-0.16 mg/mL.
For immediate use, infuse within 60 minutes of reconstitution; refrigerate for delayed use and administer within 6 hours. Administer Amondys 45 as a 30-minute IV infusion via a central venous access device, and flush the catheter post-infusion. Strictly adhere to storage timelines and visually inspect products to prevent adverse events.
- The prescribed dosage of Amondys 45 is 30 milligrams per kilogram, administered once weekly via intravenous infusion lasting 35 to 60 minutes, using an in-line 0.2-micron filter.
- In the event of a missed dose, it should be administered at the earliest opportunity following the scheduled dose.
Important:
Prior to beginning Amondys 45 treatment, discuss your current medications, medical conditions, pregnancy status, or breastfeeding intentions with your healthcare provider to assess potential interactions and customize a safe treatment plan. Report any side effects promptly to your healthcare professional. Seek personalized guidance from your doctor for any concerns or questions about Amondys 45 or your treatment regimen.
Warnings & Precautions
- Amondys 45 should not be administered if you have a known allergy to Casimersen or any components of the medication. Serious allergic reactions, such as anaphylaxis and angioedema, have been reported with Casimersen use, manifesting as difficulty breathing, chest tightness, and swelling of the mouth, face, lips, or tongue.
- If you experience signs of hypersensitivity, it’s important to seek immediate medical attention. Your doctor will manage any allergic reactions accordingly, which may involve adjusting or stopping the Amondys 45 infusion. Continuous monitoring will be conducted until the allergic reaction resolves.
- While the clinical studies didn’t show kidney toxicity, it’s crucial to monitor kidney function closely. Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio should be assessed before treatment.
- Consider measuring glomerular filtration rate. Monitor urine dipstick monthly and serum cystatin C and urine protein-to-creatinine ratio every three months during treatment. Use urine free of excreted Amondys 45 for protein monitoring. If serum cystatin C or proteinuria increases persistently, refer to a pediatric nephrologist for evaluation.
- Avoid urine protein tests using the pyrogallol red reagent to prevent false positives.
Common Amondys 45 Side Effects:
- Upper respiratory tract infection (sore throat, runny or stuffy nose, pain with swallowing)
- Cough
- Pyrexia (fever)
- Headache
- Arthralgia (joint pain)
- Oropharyngeal pain
Use in Specific Population
- Data regarding the use of Amondys 45 during pregnancy and its impact on milk production or its presence in breast milk are currently unavailable, both in human and animal studies.
- The decision to use Amondys 45 in breastfeeding mothers should consider the potential benefits of breastfeeding alongside the clinical necessity for treatment and any possible risks to the infant from either the medication or the mother’s underlying condition.
- It is indicated for treating Duchenne muscular dystrophy (DMD) in patients with a confirmed mutation amenable to exon 45 skipping, including pediatric patients. Since DMD primarily affects children and young adults, there is no experience in geriatric DMD patients.
- No specific dosage adjustments are recommended for DMD patients with renal impairment based on the estimated glomerular filtration rate, but close monitoring is advised for those with known renal function impairment during the treatment.
Storage and Handling
- Store Amondys 45 at 2°C to 8°C (36°F to 46°F)
- Keep the medicine protected from moisture.
- Do not freeze the medicine.
- Keep the medicine away from pets and children.
Our pharmaceutical procurement process is carefully organized, comprising four essential stages to guarantee a smooth and efficient experience:
- Inquiry: Our dedicated Named Patient Access Program ensures swift responses to medication inquiries, typically within 24 hours, offering comprehensive assistance.
- Verification: Sansfro ensures the availability and authorization of medications, particularly for patients seeking drugs not easily accessible in their home countries. We meticulously validate prescriptions and medical information with precision, strictly adhering to compliance standards.
- Procurement: Following thorough verification, our team utilizes our extensive supplier network to obtain the necessary medication. We negotiate for competitive quotes and manage the efficient processing of orders to ensure seamless procurement.
- Secure Dispatch: After finalizing the quote, we seamlessly coordinate the secure dispatch of your medication. Our logistics specialists are on hand to track consignments. Upholding the integrity of medication provision, we strictly follow Standard Operating Procedures within the Named Patient Import Program.
For smooth medication importation, patients are required to submit the following documentation:
- A valid prescription copy.
- Identification records.
- Details of the primary healthcare provider.
- Current residential address.
Upon receipt of all necessary documents, the Sansfro team promptly begins the application process for the import license. This license is essential for acquiring the required medication, pending government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What is Amondys 45 Sarepta?
Amondys 45, developed by Sarepta Therapeutics, is a medication for Duchenne muscular dystrophy (DMD) in patients with a specific genetic mutation. It facilitates exon 45 skipping to restore dystrophin protein production.
How should patients be monitored while taking Amondys 45?
Patients should be monitored for kidney function before and during treatment, with assessments such as serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio. Regular monitoring for signs of hypersensitivity and allergic reactions is also recommended.
How is Amondys 45 administered?
Amondys 45 is administered via intravenous infusion, typically once weekly, at a dosage of 30 milligrams per kilogram. This drug should be injected by the healthcare professional. Consult your doctor for more information.
What are the common adverse reactions associated with Amondys 45?
The most common adverse reactions (with an incidence greater than 20% and at least 5% higher than placebo) include upper respiratory tract infection, cough, pyrexia (fever), headache, arthralgia (joint pain), and oropharyngeal pain. If you experience any side effects consult your doctor immediately.
What is the Amondys 45 price in India?
For accurate pricing information on Amondys 45 in the Indian market, various factors such as import duties, taxes, exchange rates, currency fluctuations, and supply-demand dynamics must be considered. To obtain precise pricing details tailored to your needs, we recommend contacting our Patient Support Team at (+91) 93157 05373 or via email at help@sansfro.com. Our experienced team is dedicated to providing personalized assistance and reliable information to effectively address your inquiries. Please feel free to reach out to us for any assistance regarding the price of Amondys 45 in India.
How can I buy Amondys 45 online?
If you’re contemplating purchasing Amondys 45 online, particularly if it’s exclusively available in the US and Europe, we advise reaching out to the Sansfro Health team or other reputable firms with expertise in importing medications from these regions. This ensures a dependable and safe procurement procedure. Seeking guidance from experienced professionals is essential, and Sansfro Health is a trusted organization committed to facilitating access to genuine pharmaceutical products.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.