About Strensiq:
- Strensiq is a tissue nonspecific alkaline phosphatase and contains Asfotase alfa as an active substance and is designated as an orphan drug.
- HPP is a rare inherited disease that leads to early loss of teeth, malformed bones, frequent bone fractures, and difficulty breathing.
- It is caused by a TNSALP enzyme activity deficiency, which leads to elevations of several substrates like inorganic pyrophosphate (PPi).
- Elevated levels of PPi block hydroxyapatite crystal growth which inhibits bone mineralization and causes an accumulation of unmineralized bone matrix which results in rickets and bone deformation in infants and children and as osteomalacia along with muscle weakness.
- Replacement of the TNSALP enzyme following Strensiq treatment reduces the enzyme-substrate levels.
Strength:
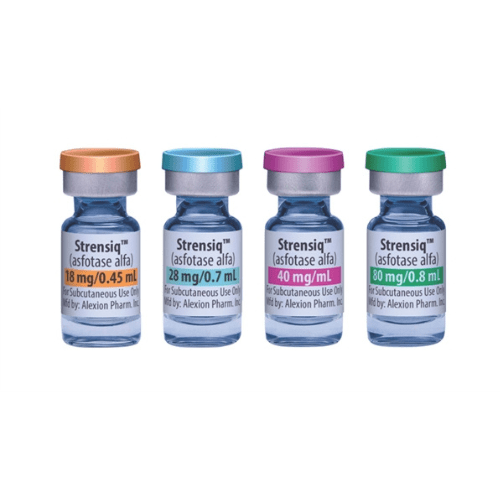
Strensiq injection is available in 18 mg/0.45 mL, 28 mg/0.7 mL, 40 mg/mL, or 80 mg/0.8 mL solution in single-use vials.
Recommended Dosage:
- The recommended dosage regimen of Strensiq for the treatment of perinatal/infantile-onset HPP is 6 mg/kg per week subcutaneously administered as:
- 2 mg/kg three times per week
- 1 mg/kg six times per week
- The dose may be increased for lack of efficacy up to 9 mg/kg per week subcutaneously as 3 mg/kg three times per week.
Important:
- Strensiq should be administered within 1 hour after removal of the vial from refrigeration.
- Rotate the injection from the abdominal area, thigh, or deltoid to reduce the risk of lipodystrophy.
- Reddened, inflamed, or swollen areas should be avoided.
- Strensiq vials are single-use only so, unused products should be discarded.
Warnings & Precautions:
- Hypersensitivity Reactions: Monitor and if a severe reaction occurs, discontinue treatment and initiate appropriate medical treatment.
- Lipodystrophy: Localized reactions were reported after several months of treatment; follow proper injection technique and rotate injection sites.
- Ectopic Calcifications (eye and kidneys): Monitor using ophthalmologic examinations and renal ultrasounds at baseline and periodically during treatment.
Common Strensiq Side Effects
- Injection site reactions
- Lipodystrophy
- Ectopic calcifications
- Hypersensitivity reactions
Use in Specific Population
Pregnancy: No available human data is available on Strensiq use in pregnant women to inform drug-associated risk.
Pediatric use: The safety and effectiveness of have been established in pediatric patients.
Storage and Handling
- Strensiq vials must be stored refrigerated conditions at 2°C to 8°C (36°F to 46°F) and protected from light.
- If removed from refrigeration, it should be administered within 1 hour.
- It should not be used beyond the expiry date.
Vials should not be shaken and frozen and for single-use only.
For the medicine procurement, we follow a simple four-step process.
- Enquiry about the medicine: When you request information about the medication you require, we will handle your data. Our Named Access Program Support team will contact you within 24 hours to assist.
- Verification Process: In our mission to help patients access medications that may not be approved or readily accessible in their home countries, Sansfro ensures the verification of medicine availability and approval. Additionally, we thoroughly review the patient’s prescription and medical information for accuracy and compliance.
- Sourcing the Medicine: Upon successfully completing the verification process, our team will initiate contact with our network of suppliers to source the required medication for you. Subsequently, our team works diligently to obtain the most favorable quotes for your medicines and oversees the processing of your order.
- Safe Delivery: We will coordinate the secure delivery of your consignment upon approval of the final quote. Our team of logistics specialists is available to provide consignment tracking assistance. Acknowledging that the Named Access Program industry is susceptible to unauthorized channels is essential. To uphold the safe and legal provision of medications, we rigorously adhere to Standard Operating Procedures.
For the importation of medication, we will require the following documents from the patient:
- An authentic prescription copy.
- A proof of identity document.
- Information about the healthcare provider in charge.
- A current residential address.
Once all these documents are provided, the Sansfro team will begin the import license application process. This license is a crucial requirement to facilitate the procurement of the necessary medication upon receiving government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What are the active ingredients of Strensiq?
Strensiq contains Asfotase alfa as an active substance and is designated as an orphan drug.
What is the mechanism of action of Strensiq?
Strensiq is a tissue nonspecific alkaline phosphatase and contains Asfotase alfa as an active substance and is designated as an orphan drug. HPP is caused by a TNSALP enzyme activity deficiency, which leads to elevations of several substrates like inorganic pyrophosphate (PPi). Elevated levels of PPi block hydroxyapatite crystal growth which inhibits bone mineralization and causes an accumulation of unmineralized bone matrix which results in rickets and bone deformation in infants and children and as osteomalacia along with muscle weakness. Replacement of the TNSALP enzyme following Strensiq treatment reduces the enzyme-substrate levels.
What are the possible serious side effects of Strensiq?
- Injection site reactions
- Lipodystrophy
- Ectopic calcifications
- Hypersensitivity reactions
Which indication Strensiq is used for?
Strensiq is a prescription medicine used for the treatment of people with perinatal, infantile, and juvenile-onset hypophosphatasia.
What is price of Strensiq in India?
For enquiry related to Strensiq price kindly reach out to our Patient Support Team at (+91) 9315705373 or help@sansfro.com

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.