About Alpelisib
Alpelisib tablets are prescribed in combination with fulvestrant for the treatment of postmenopausal women and men with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.
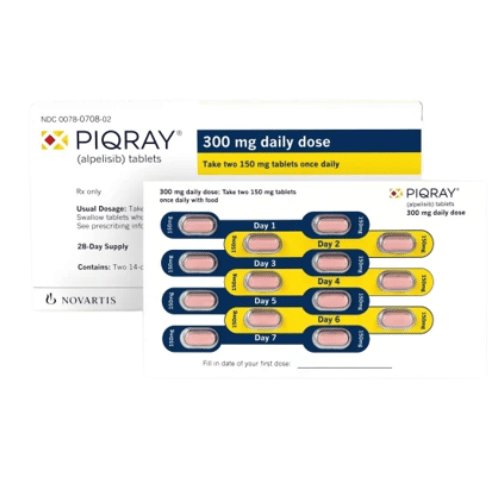
Strength
50 mg, 150 mg, and 200 mg film-coated tablets
Recommended Dosage:
The recommended dosage is two 150 mg Alpelisib tablets taken once a day with meals orally.
Important: If patients experience any adverse reactions from the Alpelisib treatment, it is necessary to consult the treating doctor.
Warnings & Precautions
- Alpelisib treatment should be discontinued permanently in cases of severe hypersensitivity. It is necessary to begin the prescribed treatment immediately.
- There have been reports of severe cutaneous responses such as Stevens-Johnson syndrome (SJS) and Erythema Multiforme (EM). If signs or symptoms of severe cutaneous responses are evident, patients must withhold the Alpelisib treatment until the cause of the reaction is confirmed by the consulting doctor. If SJS, EM, or TEN is proven, Piqray should be discontinued permanently. It is necessary to consult the treating doctor in cases of adverse reactions.
- There were reports of severe hyperglycemia, including ketoacidosis. Piqray has not been proven safe in people with Type 1 or uncontrolled Type 2 diabetes. Before beginning Alpelisib therapy, evaluate fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After starting therapy, check in regularly. As clinically appropriate, initiate or optimize anti-hyperglycemic medicines. If severe hyperglycemia arises, interrupt, reduce, or discontinue the Alpelisib tablets.
- There have been reports of severe instances of pneumonitis and interstitial lung disease. Keep an eye out for clinical signs or radiological abnormalities. If severe pneumonitis develops, interrupt or discontinue Alpelisib tablets.
- Severe episodes of diarrhea have been recorded, causing dehydration and severe renal damage. If diarrhea arises, it is advised that the patients should begin antidiarrheal medication, and increase oral fluids. If severe diarrhea occurs, interrupt, modify the dose, or discontinue Alpelisib treatment.
- Alpelisib treatment has the potential to damage a fetus (Embryo-Fetal Toxicity). Inform patients of the potential risk to a fetus and encourage them to utilize effective contraception.
Common Alpelisib Side Effects
- Increased glucose
- Increased creatinine
- Diarrhea
- Rash
- Reduced lymphocyte count
- Increased GT
- Nausea
- Increased ALT
- Fatigue
- Reduced Hemoglobin
- Increased lipase
- Reduced appetite
- Stomatitis
- Vomiting
- Reduced Weight
- Reduced Calcium
- Reduced glucose
- Prolonged aPTT
- Alopecia
Use in specific population
- Piqray poses a risk of fetal harm in pregnant women, and there is insufficient data on its use in this population. Before starting th medicine inform your doctor if you are pregnant or planning to get pregnant .
- Breastfeeding is not recommended during Piqraytreatment due to the lack of information on the presence of entrectinib or its metabolites in human milk and their impact on infants.
- Lactating women are advised to discontinue breastfeeding during treatment and for 7 days after the final dose to mitigate potential adverse reactions in breastfed children.
- Before starting Piqray, assess the pregnancy status of females of reproductive potential and recommend effective contraception during treatment and for at least 7 days post-final dose.
- Male patients with female partners of reproductive potential should also use effective contraception during treatment and for 7 days after the final dose.
- Based on animal studies, Piqray may impair fertility in males and females of reproductive potential.
- Piqray safety and effectiveness have not been established in pediatric patients, and no dose adjustment is recommended for those with mild to moderate renal impairment.
Storage and Handling
Each carton contains two blister packs with every pack containing a 14-day supply of 28 Alpelisib tablets (150 mg).
Store at 20ºC to 25ºC excursions permitted between 15ºC to 30ºC.
We follow a four-step process to procure the medicines:
- Enquiry about the medicines: You submit a request for information about the medications you require using our portal, and we process your data. Within 24 hours, a member of our Named Access Program Support will get in touch with you and provide you with all the assistance you need.
- Verification Process: As we assist patients in finding medications that are not yet approved and readily available in their home countries, Sansfro must confirm the availability and approval of the medicines they need. We also check the patient’s prescription and medical information.
- Sourcing the Medicine: After the verification process has been completed, our staff will contact our vast network of suppliers to find your medicine. Following the process, our team gets the best rates of the medicines for you and ensures processing.
- Safe Delivery: We will set up the consignment’s safe delivery after the final quote is approved. Additionally, our logistics experts can help you track your consignment. The Named Access Program industry has a large number of unauthorized channels. To provide the medications safely and legally, we do so by adhering to the Standard Operating Procedures.
When importing medication, we will need the following documents from the patient:
- A valid prescription copy.
- An identity proof document.
- Details of the attending healthcare professional.
- A current address.
Once we have all these documents, the Sansfro team will initiate the application process for an import license. This license is essential to ensure that we can obtain the necessary medication once it receives government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
How can Alpelisib help in treating breast cancer?
Piqray comprises the active component alpelisib, a targeted medication that inhibits a particular pathway known as PI3K, which is frequently hyperactive in cancer cells. Piqray tablets slow down cancer development and spread by inhibiting this route.
Who can take Piqray treatment?
Piqray treatment is suggested for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer with a PIK3CA genetic mutation. Typically, genetic testing is used to assess eligibility.
What are the potential side effects of Piqray?
Piqray often causes hyperglycemia (high blood sugar), rash, diarrhea, and elevated levels of liver enzymes. It is necessary to address any side effects with the consulting doctor and follow their recommendations.
How is Alpelisib administered?
Piqray or Alpelisib is an oral drug that comes in tablet form. It is often taken once a day with meals to enhance absorption and alleviate stomach distress. Patients must consult their doctor for recommendations based on their medical condition.
What is the price of Piqray in India?
Piqray price in India depends on the product requirement. Request more details by reaching out to our Patient Support Team at (+91) 93157 05373 or help@sansfro.com
How to buy Piqray online?
To obtain Piqray online in India, establish contact with reputable sources such as Sansfro Health or other esteemed platforms specializing in the online importation of medications. Ensure a secure transaction by seeking guidance from experienced professionals, and Sansfro Health emerges as a trustworthy choice in this field. For additional details, reach out to our dedicated Patient Support Team at (+91) 9315705373 or via email at help@sansfro.com.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.