Introduction and FDA Approval
Ultomiris (ravulizumab-cwvy) is a first-in-class, long-acting C5 inhibitor that was approved by the Food and Drug Administration (FDA) in December 2020 for the treatment of patients with Paroxysmal nocturnal hemoglobinuria PNH. Ultomiris is the brand name for ravulizumab-cwvy. The manufacturer of Ultomiris is Alexion Pharmaceuticals, Inc.
Indications and Usage
Ultomiris is indicated for the treatment of adult patients with PNH who have been previously treated with eculizumab. Ultomiris is also indicated for the treatment of adult patients with anti-CD55/CD59 antibody-positive PNH who are not eligible for eculizumab.
What is Paroxysmal Nocturnal Hemoglobinuria?
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, chronic blood disorder that affects approximately 1 in a million people. It is characterized by the destruction of red blood cells by the complement system, a part of the immune system that normally defends the body against infection. This destruction of red blood cells can lead to a variety of symptoms, including:
- Fatigue
- Anemia
- Hemoglobinuria (red urine)
- Dark urine
- Jaundice (yellowing of the skin and eyes)
- Abdominal pain
- Blood clots
The symptoms of PNH can vary from person to person and can range from mild to severe. Some people may have no symptoms at all, while others may experience severe anemia, fatigue, and blood clots. The severity of the symptoms can also fluctuate over time. There is no cure for PNH, but there are treatments that can help to manage the symptoms and prevent complications.
PNH is caused by a genetic mutation in a gene called PIG-A or PIG-T. These genes are responsible for producing proteins that protect red blood cells from being destroyed by the complement system. When these genes are mutated, the proteins are not produced, or they are not produced in the correct form. This makes red blood cells vulnerable to destruction by the complement system.
Mechanism Of Ultomiris
Ultomiris works by blocking the activation of the complement system, a part of the immune system that is responsible for the destruction of red blood cells in PNH.
Dosage and Administration
Ultomiris infusion is available as a 300 mg subcutaneous injection. The usual starting dosage is 300 mg every 8 weeks. The dosage may be adjusted as needed based on the patient’s response to treatment. Ultomiris should be injected subcutaneously into the abdomen, thigh, or upper arm.
Contraindications
ULTOMIRIS should not be used in the following cases:
- In patients with unresolved Neisseria meningitidis infection.
- History of severe allergic reactions to Ultomiris or any of its ingredients.
In patients who are not currently vaccinated against Neisseria meningitidis, unless the potential risks of delaying ULTOMIRIS treatment outweigh the risks associated with developing a meningococcal infection.
Warnings and Precautions
- Other Infections: Exercise caution when administering Ultomiris to individuals with any systemic infection.
- Infusion-Related Reactions: Monitor patients closely during infusion, interrupt for reactions, and implement appropriate supportive measures. Life-threatening meningococcal infections/sepsis have occurred in patients treated with Ultomiris and may quickly become life-threatening or fatal without early recognition and treatment.
- Follow the latest Advisory Committee on Immunization Practices (ACIP) recommendations: Especially for meningococcal vaccination in patients with HUS complement deficiencies.
- Administer meningococcal vaccines at least 2 weeks before the first dose of Ultomiris. Unless delaying Ultomiris therapy poses greater risks than the potential meningococcal infection.
- Vaccination reduces, but does not eliminate, the risk of meningococcal infection: Monitor patients for early signs of meningococcal infections and promptly evaluate if infection is suspected.
Common Side Efects Of Ultomiris
- Upper respiratory tract infection
- Headache
- Nausea
- Vomiting
- Headache
- Hypertension
- Pyrexia
Use of Ultomiris in Specific Populations
Ultomiris should be used with caution in patients with:
- Impaired liver function: Patients with impaired liver function may be more susceptible to the side effects of Ultomiris.
- Pregnancy and breastfeeding: Limited or no information is available regarding the presence of ravulizumab-cwvz in human milk, its impact on the breastfed child, or its effect on milk production. Due to the potential for serious adverse reactions in a nursing child, breastfeeding should be discontinued during treatment with ULTOMIRIS and for 8 months after the final dose.
Drug Interactions
- If ULTOMIRIS is used concurrently with Plasma Exchange, Plasmapheresis, or Intravenous Immunoglobulins, an additional dose of ULTOMIRIS may be necessary.
- Monitor closely for diminished effectiveness of ULTOMIRIS when used alongside Neonatal Fc Receptor Blockers.
Conclusion
Ultomiris is a safe and effective treatment for PNH. It is the first FDA-approved oral medication for PNH and offers several advantages over intravenous eculizumab, including a longer dosing interval and a more convenient route of administration. Ultomiris has been shown to significantly reduce the number of hemolytic events (episodes of red blood cell destruction) and improve anemia in patients with PNH.
What is Ultomiris?
Ultomiris is prescribed for paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) in patients one month and older, as well as for adult patients with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody-positive.
What are the strength of Ultomiris?
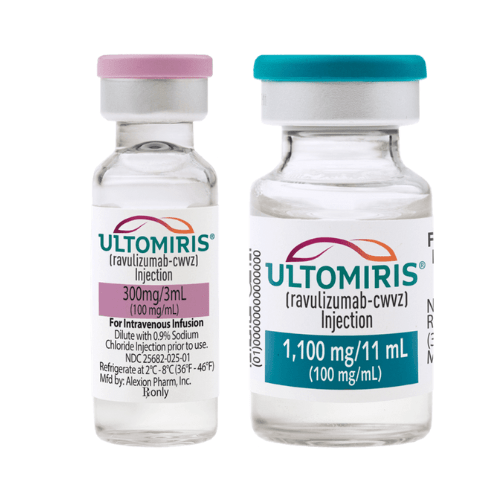
The Ultomiris injection is available in three strengths: 300 mg/30 mL (10 mg/mL), 300 mg/3 mL (100 mg/mL), and 1,100 mg/11 mL (100 mg/mL), each in a single-dose vial.
How to store Ultomiris?
Store ULTOMIRIS vials in the refrigerator at 2°C – 8°C (36°F – 46°F), keeping them in the original carton to shield from light. Ensure not to freeze and avoid shaking the vials.
How to Buy Ultomiris online?
To buy Ultomiris online, contact SANSFRO or similar pharmaceutical procurement firms specializing in medicine importation from the US and Europe. For accurate information tailored to the Indian market, reach out to the dedicated Patient Support Team at (+91) 93157 05373 or help@sansfro.com.
What is the Ultomiris price?
Several factors and product specifications contribute to the pricing of Ultomiris. For accurate and current Ultomiris price information in the Indian market, please contact our Patient Support Team at (+91) 9315705373 or via email at help@sansfro.com. Our team will provide you with the latest and precise cost details for Ultomiris.
Reference



