Piqray: Transforming the Landscape of PIK3CA-Mutated Breast Cancer Treatment
Piqray (alpelisib) is an FDA-approved kinase inhibitor for use in conjunction with fulvestrant, int…
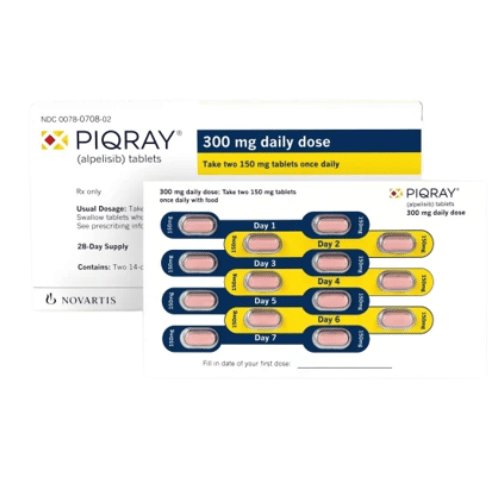
Piqray (alpelisib) is an FDA-approved kinase inhibitor for use in conjunction with fulvestrant, int…
Introduction: In recent years, the landscape of cancer treatment has witnessed a remarkable shif…

Tukysa (Tucatinib) is an antineoplastic classified as a tyrosine kinase inhibitor (TKI) and is util…
Trodelvy (sacituzumab govitecan-hziy) is prescribed for the treatment of adult patients with …

Introduction Sotyktu (deucravacitinib) is a groundbreaking oral medication for the treatment…

Introduction Rolvedon (eflapegrastim-xnst) is a prescription medication used to reduce the r…
The U.S. Food and Drug Administration approved Terlivaz for injection, making it the first and …

Introduction Relyvrio is a prescription medicine used to treat amyotrophic lateral sclerosis…
Introduction Imjudo (tremelimumab) is a type of immunotherapy drug called a checkpoint inhib…
Introduction Tecvayli (teclistamab cqyv) was approved by the FDA in December 2022 for the tr…
FDA Approval On November 14, 2022, the FDA granted accelerated approval to Elahere (mirvetux…
Introduction Tzield (teplizumab-mzwv) is a prescription medication used to delay the onset o…

Introduction Rezlidhia, developed by Metrics Contract Services, was granted FDA approval on …

Krazati, developed by Mirati Therapeutics, Inc., was granted accelerated approval by the FDA on…
On December 22, 2022, the Food and Drug Administration (FDA) granted accelerated approval to Lu…