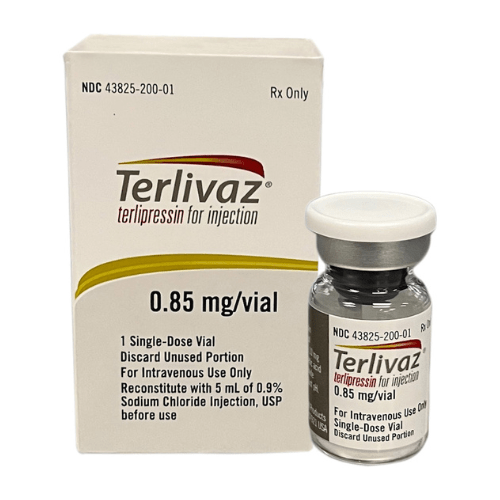
About Terlipressin
- Terlivaz received Food and Drug Administration (FDA) approval On September 14, 2022, marking it as the treatment for hepatorenal syndrome.
- Terlivaz is a vasopressin-related drug that belongs to the family called antidiuretic hormones.
- Terlivaz is prescribed as a vasopressin receptor agonist to enhance kidney function in adults diagnosed with hepatorenal syndrome, particularly in cases of rapid kidney function decline.
- Terlivaz in hepatorenal syndrome works by constricting blood vessels in the portal vein system, which reduces portal hypertension and blood flow to the liver, improving kidney function and reducing toxin release.
- Hepatorenal syndrome (HRS) refers to a serious complication of advanced liver disease, where progressive kidney dysfunction occurs. It typically arises in individuals with cirrhosis, severe alcoholic hepatitis, or other liver-related conditions.
- HRS is characterized by a rapid deterioration in kidney function, leading to renal failure.
- Symptoms may include reduced urine output, fluid retention, abdominal swelling, and potential mental confusion. HRS is considered a life-threatening condition requiring prompt medical attention.
Strength:
For injection: Terlivaz 0.85 mg (1 vial) as a lyophilized powder in a single-dose vial for reconstitution.
Recommended Dosage:
Before the initial dose, assess patients for ACLF Grade 3 and determine baseline oxygenation levels. Monitor oxygen saturation using pulse oximetry. Reconstitute each vial with 5 mL of 0.9% Sodium Chloride Injection to create a 0.85 mg/5 mL solution. Visually inspect the solution for particulate matter and discoloration before administration.
Administer Terlivaz through a peripheral or central line; a dedicated central line is not necessary. Flush the line post-administration. If not used immediately, store Terlivaz at 2°C to 8°C (36°F to 46°F) for up to 48 hours, avoiding freezing. The reconstituted solution does not require protection from light. Record the last available serum creatinine (SCr) value before treatment initiation (baseline SCr). Your doctor will determine the dosage based on your body weight and medical condition.
- During Days 1 to 3, administer Terlivaz 0.85 mg (1 vial) intravenously every 6 hours. On Day 4, assess serum creatinine (SCr) compared to baseline.
- If SCr has decreased by at least 30% from baseline, continue Terlivaz 0.85 mg (1 vial) intravenously every 6 hours.
- In case the SCr has decreased by less than 30% from baseline, consider increasing the dose to Terlivaz 1.7 mg (2 vials) intravenously every 6 hours.
- If SCr is at or above the baseline value, discontinue Terlivaz.
- Continue Terlivaz until 24 hours after two consecutive SCr values are ≤1.5 mg/dL, spaced at least 2 hours apart, or for a maximum of 14 days.
Important:
Before initiating Terlivaz injection, it’s imperative to have a comprehensive discussion about your medical history and current health status with your doctor. Regarding medications, ensure that you inform your doctor about all prescribed drugs, over-the-counter medications, and herbal supplements you are taking. Throughout the course of Terlivaz, anticipate regular monitoring of your blood pressure, oxygen levels, and kidney function.
Additionally, consider the following precautions: cease smoking, as it can exacerbate the effects of Terlivaz, and refrain from alcohol consumption to prevent potential liver damage. Should you encounter any new or worsening symptoms while on Terlivaz, promptly inform your doctor.
Warnings & Precautions
- Terlivaz has the potential to induce hypersensitivity reactions, including the severe condition of anaphylaxis. If such a reaction occurs, immediate discontinuation of Terlivaz is essential, and prompt initiation of appropriate medical treatment is warranted.
- Inform your doctor if you have serious or fatal respiratory failure (breathing problems), which is a severe condition affecting your breathing, particularly if you have excess fluid in your body or advanced liver failure.
- Your doctor will assess your oxygen levels before starting Terlivaz, and if your oxygen levels are too low (SpO2 <90%), the medication will not be initiated.
- Throughout treatment, your doctor will monitor your oxygen levels, and if they fall below 90%, Terlivaz will be stopped.
- Additionally, if you are on the list for a liver transplant, adverse reactions to Terlivaz may impact your eligibility for the transplant. It’s crucial to keep your doctor informed about your status.
- Terlivaz can also potentially cause ischemic events, affecting blood flow to various body parts. Your doctor may need to adjust or discontinue the dose accordingly.
- Always maintain open communication with your doctor regarding your health conditions and any concerns you may have.
Common Terlivaz Side Effects:
- Belly pain
- Nausea
- Diarrhea
- Headache
- Fatigue
- Slow heart rate
- Shortness of breath
- Respiratory failure
- Liver damage
- Low blood pressure
- Seizures
Use in Specific Population
- Before starting the treatment with this medicine, inform your doctor if you are pregnant or plan to become pregnant. Terlivaz may cause harm to the fetus when it is administered to a pregnant woman.
- Notify your doctor if you are breastfeeding or plan to breastfeed because it is unknown whether Terlivaz passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking Terlivaz.
- Terlivaz safety and effectiveness remain unestablished in pediatric patients. No significant differences were noted in comparison to younger subjects.
- Based on reported experiences, older patients and younger ones have shown similar responses to Terlivaz. However, it’s essential to note that some older individuals might be more sensitive to the medication.
- No dose adjustment is required in patients with hepatic impairment.
Storage and Handling
Before handling Terlipressin injections, ensure your hands are clean by washing them with soap and water.
- Keep Terlivaz vials refrigerated in the carton between 2°C to 8°C (36°F to 46°F).
- Store in the original carton to shield from light before reconstitution.
- Protect Terlivaz from light and moisture.
- Do not store Terlivaz in the bathroom or in other areas where it may be exposed to heat or humidity.
- Keep Terlivaz out of the reach of children and pets.
Our procurement process for medicines is structured around four straightforward steps:
- Enquiry: When you inquire about a medication, our Named Access Program Support team will promptly handle your request and reach out to assist you within 24 hours.
- Verification: Sansfro ensures the availability and approval of medicines, with a focus on patients seeking medications not readily accessible in their home countries. We meticulously verify prescriptions and medical information for accuracy and compliance.
- Sourcing: Following successful verification, our team leverages its network of suppliers to source the required medication. We negotiate favorable quotes and oversee the seamless processing of orders.
- Safe Delivery: Upon final approval of the quote, we efficiently coordinate the secure delivery of your medication. Our logistics specialists are available for consignment tracking. To uphold the integrity of medication provision, we strictly adhere to Standard Operating Procedures in the Named Access Program industry.
To facilitate the importation of medication, we require the following documentation from the patient:
- valid prescription copy.
- Identification record.
- Information about the primary healthcare provider.
- Current residence address.
Once all necessary documents are provided, the Sansfro team promptly initiates the import license application process. Upon government approval, this license becomes a crucial prerequisite for procuring the required medication.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What are the benefits of using Terlivaz?
- Improves kidney function in patients with HRS.
- May delay the need for a liver transplant.
- Reduces the risk of death from HRS.
Who should not take Terlivaz?
Terlivaz is not recommended for individuals with a history of a severe allergic reaction to terlipressin or any of its ingredients, those currently experiencing an active infection, individuals with coronary artery disease, a history of heart attack or stroke, or those with uncontrolled high blood pressure. If you fall into any of these categories, it is crucial to consult with your healthcare provider before considering Terlivaz.
How long can I take Terlivaz?
Terlivaz should be continued until your kidney function has improved and stabilized. The maximum recommended duration of treatment is 14 days. However, your healthcare provider will decide the duration based on your condition.
What is Terlivaz used for?
Terlivaz is a vasopressin receptor agonist indicated to improve kidney function in adults with hepatorenal syndrome with a rapid reduction in kidney function.
Is Terlivaz suitable for pediatric patients?
The safety and effectiveness of Terlivaz have not been established in pediatric patients.
What is the price of Terlivaz in India?
Terlivaz price in India depends on the product requirement. Request more details by contacting our Patient Support Team at (+91) 9315705373 or help@sansfro.com.
How can I buy Terlivaz online?
If you’re looking to buy Terlivaz online, it’s advisable to reach out to the Sansfro Health team or other reputable organizations with expertise in importing medications from these regions. Make sure you have proper prescription with you.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.