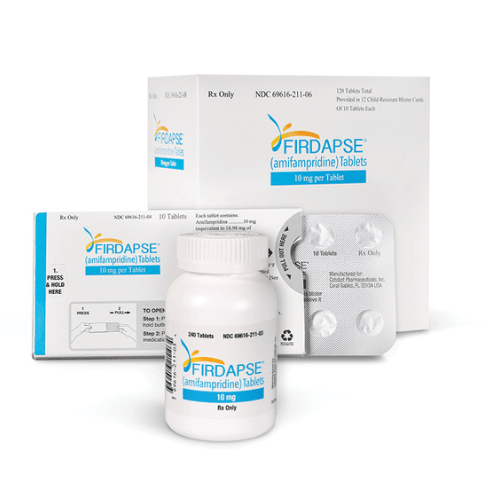
About Firdapse
- Firdapse received Food and Drug Administration (FDA) approval on November 28, 2018, marking it as the first FDA-approved treatment for LEMS.
- Firdapse is an oral medication which belongs to the family called a potassium channel blocker.
- It is used to treat LEMS in adults and pediatric patients aged six and older.
- LEMS is a rare autoimmune disorder. In LEMS, the immune system mistakenly attacks the neuromuscular junction, disrupting the communication between nerve cells and muscle cells.
- This condition is most frequently associated with cancer, particularly small cell lung cancer, often appearing before or alongside the cancer diagnosis. However, it can also be linked to other autoimmune diseases.
- Firdapse tablet, meant for oral use, contain 10mg of Amifampridine (equivalent to 18.98mg amifampridine phosphate) along with inactive components such as calcium stearate, colloidal silicon dioxide, and microcrystalline cellulose.
Strength:
Galafold tablets are delivered in a strength of 10mg respectively, for oral consumption.
Recommended Dosage:
To ensure proper use of Firdapse, follow your doctor’s prescribed regimen, and if your dose is less than 5mg or you have difficulty swallowing tablets, consult the detailed Instructions for Use to prepare a suspension. Do not alter your dose without your doctor’s guidance, and if you miss a dose, skip it and take the next scheduled dose without doubling up.
Firdapse tablets can be split if needed and can be taken with or without food. Avoid combining with other medications that increase seizure risk, and in the case of an overdose, promptly contact your doctor or seek emergency medical attention at the nearest hospital.
- Administer this medication orally in divided doses, usually 3 to 4 times daily. The initial recommended dosage for adults and pediatric patients weighing 45kg or more is between 15mg to 30mg per day, split into multiple doses.
- You can increase the dosage by 5mg daily every 3 to 4 days, with a maximum single dose of 20mg and a daily maximum of 80mg.
- For pediatric patients under 45kg, start with 5mg to 15mg daily, divided into doses, and increase by 2.5mg daily every 3 to 4 days.
- In cases of renal impairment (CLcr 15 to 90 mL/min), start with the lowest recommended daily dosage.
- The maximum single dose is 10mg, and the daily maximum is 40mg. Adjust the initial daily dosage for patients with renal impairment, hepatic impairment, or known N-Acetyltransferase 2 (NAT2) poor metabolizers to the lowest recommended level.
- If necessary, a 1mg/mL suspension can be prepared for patients who need dose adjustments less than 5mg increments or have difficulty swallowing or require a feeding tube.
Important:
Make sure not to miss any Firdapse doses. If you do miss one, take it as soon as possible, but if your next dose is near, skip the missed dose and stick to your regular schedule; never double up. Keep your doctor informed about all your medications, including over-the-counter drugs, herbs, and vitamins, as some may interact with Firdapse, potentially causing severe side effects. If you have questions or concerns about this medication, discuss them with your doctor. Following these instructions diligently is essential to maximize the medicine’s benefits and minimize the risk of side effects.
Warnings & Precautions
- Firdapse has the potential to induce hypersensitivity reactions, including the severe condition of anaphylaxis. If such a reaction occurs, immediate discontinuation of Firdapse is essential, and prompt initiation of appropriate medical treatment is warranted.
- This medication should not be administered to individuals who have demonstrated hypersensitivity to amifampridine phosphate, other aminopyridines, or any of the tablet’s inactive ingredients.
- Furthermore, Firdapse should be avoided in patients with a history of seizures, as it may trigger seizure activity. Physicians should carefully consider discontinuation or dosage reduction of Firdapse in patients who experience seizures during treatment.
- Exercise caution when prescribing Firdapse to patients with renal or hepatic impairment. No specific dosage recommendation is available for patients with end-stage renal disease.
- Before consuming this medicine inform your doctor if you are pregnant or plan to become pregnant. It is not known if Firdapse will harm your unborn baby. You and your doctor will decide if you should take Firdapse while you are pregnant.
- Notify your doctor if you are breastfeeding or plan to breastfeed because it is unknown whether Firdapse passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking Firdapse.
- Caution is advised when using Firdapse with drugs that lower seizure threshold, as it may increase the risk of seizures. Additionally, the use of drugs with cholinergic effects, such as cholinesterase inhibitors, alongside Firdapse may enhance cholinergic effects and raise the potential for adverse reactions.
Common Firdapse Side Effects:
- Shortness of breath or trouble breathing
- Swelling of your throat or tongue
- Hives
- Tingling around the mouth, tongue, face, fingers, toes, and other body parts
- Upper respiratory infection
- Stomach pain
- Nausea
- Diarrhea
- Headache
- Increased liver enzymes
- Back pain
- High blood pressure
- Muscle spasms
Use in Specific Population
- Firdapse is safe and effective for treating LEMS in pediatric patients aged 6 and older, supported by various evidence sources. Its use in children under 6 is not established.
- Clinical studies in elderly individuals were limited, but available data did not show significant differences in responses compared to younger patients.
- However, when dosing elderly patients, caution is recommended, typically starting at the lower end of the dosing range due to potential age-related factors.
Storage and Handling
Before handling Galafold tablets, ensure your hands are clean by washing them with soap and water. Avoid touching the tablet with wet hands. If you accidentally touch a tablet with wet hands, make sure your hands are completely dry before handling the tablet again.
For the medicine procurement, we follow a simple four-step process.
- Enquiry about the medicine: When you request information about the medication you require, we will handle your data. Our Named Access Program Support team will contact you within 24 hours to assist.
- Verification Process: In our mission to help patients access medications that may not be approved or readily accessible in their home countries, Sansfro ensures the verification of medicine availability and approval. Additionally, we thoroughly review the patient’s prescription and medical information for accuracy and compliance.
- Sourcing the Medicine: Upon successfully completing the verification process, our team will initiate contact with our network of suppliers to source the required medication for you. Subsequently, our team works diligently to obtain the most favorable quotes for your medicines and oversees the processing of your order.
- Safe Delivery: We will coordinate the secure delivery of your consignment upon approval of the final quote. Our team of logistics specialists is available to provide consignment tracking assistance. Acknowledging that the Named Access Program industry is susceptible to unauthorized channels is essential. To uphold the safe and legal provision of medications, we rigorously adhere to Standard Operating Procedures.
For the importation of medication, we will require the following documents from the patient:
- An authentic prescription copy.
- A proof of identity document.
- Information about the healthcare provider in charge.
- A current residential address.
Once all these documents are provided, the Sansfro team will begin the import license application process. This license is a crucial requirement to facilitate the procurement of the necessary medication upon receiving government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
Is Firdapse a cure for LEMS?
Firdapse is not a cure for LEMS but is used to manage and improve muscle function in patients with the condition. Consult your healthcare professional for more information.
Is a prescription required to obtain Firdapse?
Yes, Firdapse is a prescription medication, and patients must obtain it through a healthcare provider’s prescription.
What is the function and class of Firdapse?
Firdapse belongs to the drug class known as “Cholinergic muscle stimulants.” It functions by blocking potassium channels in nerve endings, which helps improve the communication between nerves and muscles, particularly in patients with Lambert-Eaton myasthenic syndrome (LEMS). This action enhances the transmission of nerve signals to the muscles, leading to improved muscle function in individuals with LEMS.
What if I miss the dose of Firdapse?
If you miss a dose of Firdapse, take it as soon as possible. If it’s close to your next scheduled dose, skip the missed one and continue as usual. Don’t double the dose. Follow your doctor’s instructions for the best results and consult them with any concerns or questions.
Are Firdapse and Amifampridine different medications?
Firdapse and amifampridine are not different medications in terms of their active ingredient. Firdapse contains amifampridine phosphate as its active ingredient. Amifampridine is the generic name for the active compound, while Firdapse is a brand name for a specific formulation of amifampridine phosphate. In essence, Firdapse and amifampridine refer to the same medication, with Firdapse being a specific brand of amifampridine.
What is the price of Firdapse in India?
Firdapse price in India depends on the product requirement. Request more details by contacting our Patient Support Team at (+91) 93157 05373 or help@sansfro.com.

Dr Anchal Aryan
Medical counselor
Patient Stories
Reference
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.