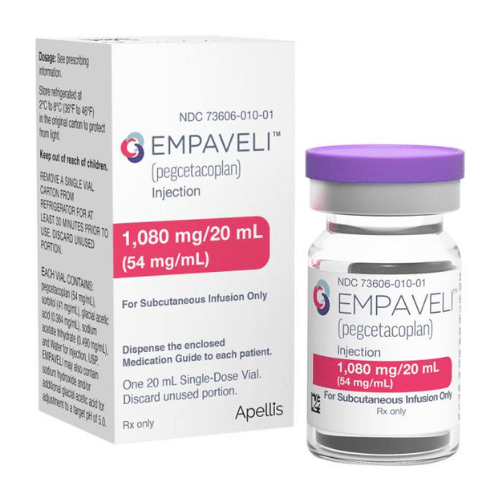
About Pegcetacoplan
- Pegcetacoplan, marketed as Empaveli, received its initial FDA approval on May 14, 2021, for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
- Empaveli, a complement inhibitor, is indicated for adult patients with PNH. Pegcetacoplan functions by binding to complement protein C3 and its activation fragment C3b, thereby regulating the cleavage of C3 and the subsequent generation of downstream effectors of complement activation.
- In PNH, extravascular hemolysis (EVH) is facilitated by C3b opsonization, while intravascular hemolysis (IVH) is mediated by the downstream membrane attack complex (MAC).
- Pegcetacoplan operates proximally in the complement cascade, controlling both C3b-mediated EVH and terminal complement-mediated IVH.
- Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematological disorder characterized by abnormal red blood cell destruction due to a mutation in the PIG-A gene, leading to deficiency in certain surface proteins.
- This deficiency renders blood cells susceptible to immune system attack, resulting in hemolytic anemia, fatigue, dark urine, abdominal pain, and blood clots.
Strength:
Empaveli injection of 1,080 mg/20 mL (54 mg/mL) in a single-dose vial.
Recommended Dosage:
Patients should receive vaccination against encapsulated bacteria, such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B, at least 2 weeks before starting the therapy. If the treatment needs to begin immediately and vaccines were given less than 2 weeks prior, patients should also receive 2 weeks of antibacterial drug prophylaxis.
- The recommended dosage of Empaveli is 1,080 mg administered via subcutaneous infusion twice weekly using a commercially available infusion pump with a reservoir of at least 20 mL.
For patients transitioning to Empaveli from C5 inhibitors to minimize the risk of hemolysis upon abrupt treatment cessation:
- For those switching from eculizumab, initiate Empaveli while maintaining eculizumab at its current dosage. After 4 weeks, discontinue eculizumab before continuing solely with the therapy.
- For patients switching from ravulizumab, begin the treatment no later than 4 weeks after the last ravulizumab dose.
In the event that lactate dehydrogenase (LDH) levels surpass twice the upper limit of normal (ULN), it is recommended to modify the dosing regimen to 1,080 mg every three days. Additionally, when considering an increase in dosage, it is crucial to monitor LDH levels closely, conducting assessments twice weekly for a minimum duration of 4 weeks. This proactive approach to dosage adjustment and monitoring helps ensure optimal patient safety and efficacy during the therapy.
Important:
Empaveli should be administered under healthcare professional guidance, with patients or caregivers administering after proper training. Aseptic technique is necessary, and the medication should be allowed to reach room temperature before use. Visual inspection for particulate matter and discoloration is required, and infusion sites should be rotated. Infusion times vary based on the number of sites used. Unused portions should be discarded.
Before starting treatment, inform your doctor about your pregnancy status, plans for pregnancy, or breastfeeding. Disclose all medical conditions and current medications, including prescriptions, over-the-counter drugs, vitamins, and herbal supplements. This helps your doctor assess potential interactions and personalize your treatment plan.
Warnings & Precautions
- Empaveli is contraindicated in patients with known hypersensitivity to Pegcetacoplan or any of the ingredients in the formulation.
- Additionally, individuals who have not received vaccination against specific encapsulated bacteria should not use Empaveli, unless delaying treatment poses greater risks than the potential development of a bacterial infection with an encapsulated organism.
- Moreover, This medicine should not be administered to patients with unresolved severe infections caused by encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae.
- Patients treated with Empaveli are at risk of meningococcal infections, which can rapidly become life-threatening if not promptly recognized and treated.
- Empaveli use may increase susceptibility to serious infections, particularly those caused by encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitidis types A, C, W, Y, and B, and Haemophilus influenzae type B.
- It is essential to adhere to the latest Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against encapsulated bacteria.
- Patients should be vaccinated against these bacteria at least 2 weeks before starting the medicine unless the potential risks of delaying therapy outweigh the risks of developing a serious infection.
- While vaccination reduces the risk of serious infections, it does not eliminate it entirely.
- Healthcare providers should monitor patients closely for early signs of serious infections and promptly evaluate if infection is suspected.
- Caution should also be exercised when administering the medicine to patients with serious infections caused by encapsulated bacteria, and infusion-related reactions and potential interference with laboratory tests, particularly coagulation panels, should be carefully monitored and managed as necessary.
Common Empaveli Side Effects:
- Injection-site reactions
- Infections
- Diarrhea
- Abdominal pain
- Respiratory tract infection
- Pain in extremity
- Hypokalemia
- Fatigue
- Viral infection
- Cough
- Arthralgia
- Dizziness
- Headache
- Rash
Use in Specific Population
- Insufficient data are available regarding the use of Empaveli in pregnant women to determine its potential association with major birth defects, miscarriage, or adverse outcomes for both the mother and fetus.
- Untreated paroxysmal nocturnal hemoglobinuria (PNH) during pregnancy poses risks to both the mother and the fetus. It is uncertain whether pegcetacoplan, the active ingredient in Empaveli, is excreted in human breast milk or if it can be absorbed by nursing infants.
- While pegcetacoplan injection has been detected in the milk of lactating monkeys in animal studies, its effects on milk production in humans are unknown.
- Considering that many medications are excreted in breast milk and the potential for serious adverse reactions in breastfeeding infants, breastfeeding should be discontinued during the treatment and for 40 days after the final dose.
- Female patients of reproductive age should undergo pregnancy testing before starting the treatment and should use effective contraception during treatment and for 40 days after the last dose to prevent potential embryo-fetal harm.
- The safety and efficacy in pediatric patients have not been established, and there is insufficient clinical data on its use in individuals aged 65 and older to determine if they respond differently from younger patients.
- While clinical studies do not include enough elderly subjects, reported clinical experiences have not identified any notable differences in responses between geriatric and younger patients.
Storage and Handling
- Keep Empaveli stored in its original carton in a refrigerator at a temperature between 2°C to 8°C (36°F to 46°F) to shield it from light exposure. Avoid freezing the medication.
- Do not freeze the medicine.
- Keep the medicine away from pets and children.
Our pharmaceutical procurement process is carefully organized, comprising four essential stages to guarantee a smooth and efficient experience:
- Inquiry: Our dedicated Named Patient Access Program ensures swift responses to medication inquiries, typically within 24 hours, offering comprehensive assistance.
- Verification: Sansfro ensures the availability and authorization of medications, particularly for patients seeking drugs not easily accessible in their home countries. We meticulously validate prescriptions and medical information with precision, strictly adhering to compliance standards.
- Procurement: Following thorough verification, our team utilizes our extensive supplier network to obtain the necessary medication. We negotiate for competitive quotes and manage the efficient processing of orders to ensure seamless procurement.
- Secure Dispatch: After finalizing the quote, we seamlessly coordinate the secure dispatch of your medication. Our logistics specialists are on hand to track consignments. Upholding the integrity of medication provision, we strictly follow Standard Operating Procedures within the Named Patient Import Program.
Documentation Requirements:
For smooth medication importation, patients are required to submit the following documentation:
- A valid prescription copy.
- Identification records.
- Details of the primary healthcare provider.
- Current residential address.
Upon receipt of all necessary documents, the Sansfro team promptly begins the application process for the import license. This license is essential for acquiring the required medication, pending government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
Can I take Empaveli if I have allergies?
No, do not take this medicine if you are allergic to pegcetacoplan or any of the ingredients in Empaveli. Consult your healthcare provider for alternative treatment options.
Can Empaveli be taken during pregnancy or breastfeeding?
Empaveli may harm an unborn baby, so inform your healthcare provider if you are pregnant or planning to become pregnant. Pregnant women should have a pregnancy test before starting the treatment. It is not known if it passes into breast milk, so breastfeeding is not recommended during treatment and for 40 days after the last dose.
Why is vaccination important before taking Empaveli?
Vaccination against Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B is necessary before starting Empaveli treatment to reduce the risk of serious infections. Consult your healthcare provider for vaccination recommendations and guidance.
What is the Empaveli price in India?
Determining the cost of Empaveli in the Indian market involves considering various factors such as import duties, taxes, exchange rates, currency fluctuations, and supply and demand conditions. For detailed pricing information tailored to your specific needs, we recommend contacting our Patient Support Team at (+91) 93157 05373 or via email at help@sansfro.com. Our dedicated specialists are committed to offering personalized assistance and accurate information to address your inquiries effectively.
How can I buy Empaveli online?
If you’re contemplating buying Empaveli online, particularly if it’s solely accessible in the US and Europe, we advise reaching out to the Sansfro Health team or other reputable firms specializing in drug imports from these regions. This ensures a dependable and safe procurement process. Seeking advice from experts is essential, and Sansfro Health is a reliable entity committed to facilitating access to genuine pharmaceuticals.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.