About Anifrolumab-fnia
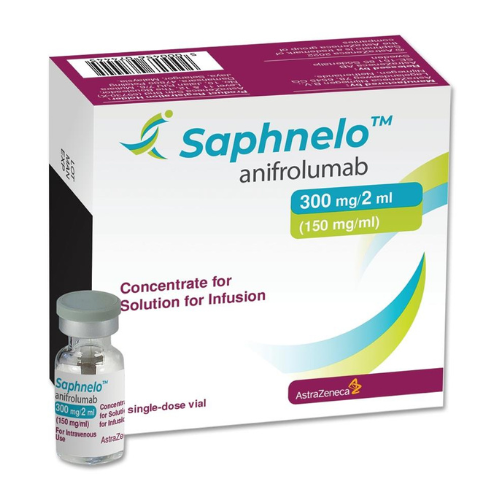
- Anifrolumab-fnia, sold under the brand name Saphnelo.
- Anifrolumab, initially approved by the U.S. Food and Drug Administration (FDA) on July 30, 2021, represents a significant advancement in the treatment landscape for systemic lupus erythematosus (SLE). This medication is specifically indicated for adult patients grappling with moderate to severe SLE who are already receiving standard therapy. Its mechanism of action is rooted in its function as a type I interferon (IFN) receptor antagonist.
- Saphnelo, the active component in Anifrolumab, binds to subunit 1 of the type I interferon receptor, effectively blocking the activity of type I interferons associated with heightened disease activity and severity in SLE patients.
- The approval of Anifrolumab marks a crucial milestone in the management of SLE, a complex autoimmune disorder characterized by systemic inflammation and a range of clinical manifestations.
- Prior to the availability of Anifrolumab, treatment options for SLE were limited, often focusing on symptom management and immunosuppressive therapies.
Strength:
The medication is provided in the form of a single-dose vial containing Saphnelo 300 mg/2 mL (150 mg/mL) for intravenous injection.
Recommended Dosage:
Your healthcare provider will administer the drug intravenously, typically through a needle inserted into a vein. The infusion process typically takes around 30 minutes to deliver the full dose.
- The recommended dosage of Saphnelo infusion is 300 mg, administered intravenously over 30 minutes, once every 4 weeks.
- If a scheduled infusion is missed, promptly administer the dose as soon as possible. However, it is crucial to maintain a minimum interval of 14 days between infusions.
Important:
Prepare the diluted Saphnelo infusion solution using an aseptic technique. Inspect the vial for contamination; discard it if cloudy or discolored. Withdraw 2 mL of the drug and add to a 100 mL bag of 0.9% Sodium Chloride Injection, USP.
Mix gently and use immediately or store for up to 24 hours refrigerated. Administer intravenously over 30 minutes with a sterile filter line. Flush the line after infusion. Dispose of unused products and waste properly.
Warnings & Precautions
- Saphnelo should not be used in patients with a known history of anaphylaxis to anifrolumab-fnia.
- Serious infections, including respiratory infections and herpes zoster, can occur with the medicine. Avoid starting treatment during an active infection and consider interrupting therapy if a new infection develops.
- Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with this medicine.
- Consider individual benefit-risk profiles in patients with known risk factors for malignancy before prescribing.
- Avoid live or live-attenuated vaccines in patients on this drug. Additionally, it is not recommended to use Saphnelo with other biologic therapies.
Common Saphnelo Side Effects:
- Nasopharyngitis
- Upper respiratory tract infections
- Bronchitis
- Infusion-related reactions
- Herpes zoster
- Cough
Use in Specific Population
- Limited human data on the use of Saphnelo in pregnant women are insufficient to assess the risk of major birth defects, miscarriage, or adverse outcomes for both mother and fetus.
- It’s important to note that all pregnancies carry a background risk of such outcomes.
- There is currently no data available regarding the presence of Saphnelo in human milk, its effects on breastfeeding infants, or its impact on milk production.
- Studies in female cynomolgus monkeys administered anifrolumab-fni detected the presence of anifrolumab-fnia in their milk.
- The safety and effectiveness of Saphnelo in pediatric patients under 18 years old have not been established.
Storage and Handling
- Store vials of Saphnelo in the refrigerator at temperatures between 36°F and 46°F (2°C and 8°C).
- Ensure not to use the medicine past the expiration date indicated on the vial.
- Do not freeze the medicine.
- Keep the medicine away from pets and children.
Our pharmaceutical procurement process is meticulously structured, encompassing four crucial stages to ensure a seamless and effective experience:
- Inquiry: When inquiring about a specific medication, our specialized Named Patient Access Program team responds promptly, typically assisting within 24 hours.
- Verification: Sansfro guarantees the availability and authorization of medicines, particularly for patients seeking medications not readily available in their home countries. We meticulously validate prescriptions and medical details with precision, strictly adhering to compliance standards.
- Procurement: Following successful verification, our team utilizes its extensive supplier network to procure the required medication. We engage in negotiations for favorable quotes and oversee the smooth processing of orders.
- Secure Dispatch: Upon finalizing the quote, we efficiently coordinate the secure dispatch of your medication. Our logistics specialists are available for consignment tracking. To maintain the integrity of medication provision, we strictly adhere to Standard Operating Procedures within the Named Patient Import Program.
To facilitate the smooth importation of medication, patients need to provide the following documents:
- A valid copy of the prescription.
- Identification records.
- Information about the primary healthcare provider.
- Current residence address.
Upon receipt of all necessary documents, the Sansfro team promptly initiates the application process for the import license. This license is an essential requirement for acquiring the needed medication, subject to government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What is Saphnelo and who is it prescribed for?
Saphnelo is a prescription medication for adults with moderate to severe systemic lupus erythematosus (SLE) who are already on other lupus treatments. It contains anifrolumab-fnia, a monoclonal antibody, and is used to reduce disease activity in lupus patients. However, it is not approved for severe lupus nephritis or central nervous system lupus, and its safety in those under 18 is not established/
What are the possible side effects of Saphnelo?
Saphnelo may cause serious side effects, including serious infections such as respiratory infections and shingles, allergic reactions including anaphylaxis, and an increased risk of certain cancers. Common side effects may include upper respiratory infections, infusion reactions, cough, bronchitis, and shingles. However, these are not all of the possible side effects.
What should I tell my healthcare provider before receiving Saphnelo?
Before receiving Saphnelo, ensure to disclose all your medical conditions to your healthcare provider. This includes any existing infections, scheduled vaccinations, history of cancer, and pregnancy or plans to become pregnant. Also, inform your healthcare provider if you are breastfeeding or intend to breastfeed. It’s essential to provide a comprehensive list of all medications you are currently taking, including prescriptions, over-the-counter drugs, vitamins, and herbal supplements.
How to administer Saphnelo injection?
Saphnelo injection is administered through a needle placed in a vein (IV or intravenous infusion), usually over a 30-minute period. It is typically given once every 4 weeks. If you miss an appointment, contact your healthcare provider to reschedule.
What is the Saphnelo price in India?
To determine the Saphnelo cost in the Indian market, several factors such as import duties, taxes, exchange rates, currency fluctuations, and supply and demand conditions need to be considered. For detailed pricing information, we recommend reaching out to our Patient Support Team at (+91) 93157 05373 or help@sansfro.com. Our team of specialists is dedicated to providing personalized assistance and accurate information to address your inquiries effectively.
How can I buy Saphnelo online?
If you’re considering purchasing Saphnelo online, especially if it’s only available in the US / Europe, we recommend reaching out to the Sansfro Health team or other reputable companies specializing in importing drugs from these regions. This ensures a reliable and secure procurement process. Seeking guidance from experts is crucial, and Sansfro Health is a trusted entity dedicated to facilitating access to authentic pharmaceuticals.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.