About Lonapegsomatropin-tcgd
- Skytrofa contains Lonapegsomatropin, an active ingredient classified as a human growth hormone utilized for the treatment of growth hormone deficiency.
- Lonapegsomatropin, functioning as a prodrug of somatropin, undergoes autocleavage, releasing active somatropin from the methoxypolyethylene glycol carrier.
- This compound binds to the growth hormone receptor, resulting in both direct effects and indirect effects mediated by insulin-like growth factor-1 (IGF-1).
- The impact of somatropin includes stimulation of chondrocyte differentiation and proliferation, glucose release from the liver, protein synthesis, lipolysis, and direct tissue and metabolic effects.
- In pediatric patients with growth hormone deficiency (GHD), somatropin stimulates skeletal growth by influencing the growth plates (epiphyses) of long bones.
- Lonapegsomatropin received approval for medical use in the United States in August 2021 and the European Union in January 2022.
Strength:
Skytrofa injection is available in dosages ranging from 3 mg, 3.6 mg, 4.3 mg, 5.2 mg, 6.3 mg, 7.6 mg, 9.1 mg, 11 mg, to 13.3 mg.
Recommended Dosage:
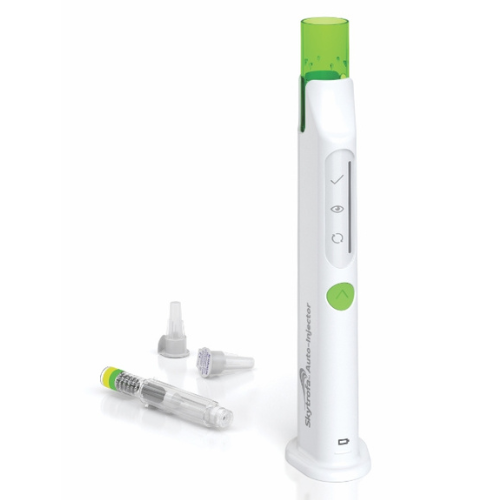
Skytrofa is administered via subcutaneous injection once weekly, Skytrofa therapy requires supervision from a physician experienced in diagnosing and managing pediatric patients with growth failure due to growth hormone deficiency (GHD). Before initiating Skytrofa treatment, conduct a fundoscopic examination to rule out preexisting papilledema, with periodic reassessments. The Skytrofa cartridge, designed exclusively for use with the Skytrofa Auto-Injector, should be kept at room temperature for 15 minutes if refrigerated. This Auto-Injector ensures a fully automated reconstitution of the lyophilized drug product, followed by a manual mixing step controlled by the device.
Upon inserting the injection needle into the skin, the device automatically delivers the drug product, aided by built-in electronics and software guiding the user through the preparation and injection sequence, confirming the full dose’s delivery. The reconstituted solution should be clear to opalescent, devoid of cloudiness or particulate matter. Utilize Skytrofa cartridges within 4 hours after reconstitution, discarding them after this period when stored at room temperature up to 86°F (30°C). Administer Skytrofa subcutaneously into the abdomen, buttock, or thigh, rotating injection sites to minimize the risk of lipoatrophy.
- For patients new to treatment and those transitioning from daily somatropin therapy, the suggested dosage of Skytrofa is 0.24 mg/kg body weight, given once weekly.
- Personalize and adjust the Skytrofa dosage according to individual responses, titrating for optimal effectiveness.
- When shifting from daily somatropin therapy to the once-weekly Skytrofa regimen, maintain a minimum 8-hour gap between the final dose of daily somatropin and the initial dose of once-weekly Skytrofa.
- Monitor compliance and investigate other potential causes of insufficient growth, such as hypothyroidism, undernutrition, advanced bone age, and antibodies to recombinant human growth hormone, particularly if patients fail to show an increase in height velocity, especially in the first treatment year.
- Cease Skytrofa administration upon the occurrence of epiphyseal fusion.
Important:
Administer any missed dose at the earliest opportunity, ensuring it is no later than 2 days after the scheduled dose. To prevent missed doses, Skytrofa can be taken 2 days before or 2 days after the planned dosing day. Resume the once-weekly dosing schedule for the next dose on the previously designated day. If more than 2 days have lapsed since the scheduled day, skip the missed dose and administer the subsequent dose on the regular day. Maintain a minimum of 5 days between doses.
Before initiating Skytrofa, promptly inform your doctor of any adverse reactions or unusual symptoms observed during treatment. Provide comprehensive details about pre-existing health conditions to ensure the safe administration of the medication. Open and transparent communication regarding your health is crucial for personalized care and addressing any concerns that may arise during treatment. Additionally, disclose information about current medications to avoid potential interactions, especially for individuals with pre-existing conditions. This ensures comprehensive care and minimizes associated risks.
Warnings & Precautions
- Patients using Skytrofa should be aware of potential risks and take necessary precautions.
- Severe hypersensitivity reactions, including anaphylaxis and angioedema, have been reported, requiring immediate medical attention in case of allergic responses.
- There is an increased risk of neoplasms, especially in patients with preexisting tumors, and childhood cancer survivors treated with somatropin may face a heightened risk of developing a second neoplasm, particularly meningiomas.
- Regular monitoring of glucose levels is advised due to the potential for glucose intolerance and diabetes mellitus, with diabetics possibly needing adjustments in concurrent antihyperglycemic drug doses.
- Intracranial hypertension may develop, usually reversible after discontinuation or dose reduction.
- Fluid retention, including edema, arthralgia, and carpal tunnel syndrome, may occur, necessitating dose reduction.
- Patients should be monitored for hypoadrenalism, hypothyroidism, and slipped capital femoral epiphysis.
- Progression of preexisting scoliosis and pancreatitis are potential concerns.
- Replacement glucocorticoid treatment may be necessary for patients with hypoadrenalism.
- Adjustments in glucocorticoid dosing are recommended to avoid inhibitory effects on growth.
- Caution is advised when using Skytrofa with cytochrome P450-metabolized drugs, oral estrogen, and insulin or other antihyperglycemic agents, with possible dose adjustments.
- Regular communication with healthcare providers is essential to address any concerns or adverse reactions promptly.
Common Skytrofa Side Effects:
- Decreased blood pressure: symptoms like dizziness, lightheadedness, or fainting.
- Nausea and vomiting: usually mild and temporary.
- Increased blood pressure.
- Difficulty sleeping.
- Anxiety.
- Loss of appetite.
- Depression.
- Numbness or tingling in hands or feet.
- Changes in taste.
- Dizziness.
- Swelling of hands, ankles, or feet.
- Fever.
- Chills.
- Skin rash.
- Hair loss.
- Difficulty breathing.
- Vision problems.
- Ear pain.
- Feeling of movement.
- Injection site reactions: redness, swelling, bruising, pain, itching, and burning at the injection site, usually mild and go away after a few days.
- Nasal congestion: runny nose, stuffy nose, and sinus pain are common.
- Muscle pain and stiffness: may worsen or occur for the first time.
- Fatigue: feeling tired or lacking energy.
- Headache: can range from mild to severe.
- High blood alkaline phosphatase: a common finding in blood tests of people taking Skytrofa and is not usually a cause for concern.
- Joint pain: pain or stiffness in joints.
- Cough: usually mild and temporary.
- Abdominal pain: can be mild and temporary.
- Diarrhea: loose or watery stools
Use in Specific Population
- Insufficient data are available concerning the use of lonapegsomatropin-tcgd in pregnant individuals to assess potential risks related to major birth defects, miscarriage, or adverse outcomes for the mother or fetus.
- Likewise, there is a lack of information regarding the presence of lonapegsomatropin-tcgd in human milk, its impact on the breastfed infant, or its effects on milk production.
- It is essential to weigh the developmental and health advantages of breastfeeding against the clinical necessity for Skytrofa and potential adverse effects on the breastfed infant arising from either Skytrofa or the underlying maternal condition.
- Notably, the safety and efficacy of Skytrofa in children under 1 year old have not been established.
Storage and Handling
- Store Skytrofa cartridges in the outer carton at a refrigerated temperature range of 36°F to 46°F (2°C to 8°C) to shield them from light until the specified expiration date.
- Avoid freezing.
- Alternatively, if Skytrofa outer cartons with blistered cartridges are used, they can be stored at room temperature [up to 86°F (30°C)] for a duration of up to 6 months.
- Return them to refrigeration within this 6-month period.
- Indicate the date of initial removal from the refrigerator in the designated space on the outer carton.
- Refrain from using Skytrofa beyond its expiration date or after 6 months from the date it was initially removed from refrigeration.
Our pharmaceutical sourcing process is meticulously designed, encompassing four essential stages to ensure a streamlined and effective experience:
- Inquiry: When seeking information about a specific medication, our dedicated Named Patient Access Program team responds promptly, typically offering assistance within 24 hours.
- Validation: Sansfro diligently verifies the availability and authorization of medicines, especially for patients needing medications not readily accessible in their home countries. We rigorously confirm prescriptions and medical details with precision, strictly adhering to compliance standards.
- Procurement: Following successful validation, our team utilizes its extensive supplier network to secure the required medication. We engage in negotiations for favorable quotes and oversee the smooth processing of orders.
- Secure Dispatch: After finalizing the quote, we efficiently coordinate the secure dispatch of your medication. Our logistics specialists are available for consignment tracking. To maintain the integrity of medication provision, we strictly adhere to Standard Operating Procedures within the Named Patient access Program industry.
To facilitate the seamless importation of medication, patients must provide the following documents:
- A valid copy of the prescription.
- Identification records.
- Information about the primary healthcare provider.
- Current residence address.
Upon receipt of all required documents, the Sansfro team promptly initiates the application process for the import license an essential prerequisite for securing the required medication, subject to government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What is Skytrofa and what is its indication?
Skytrofa, containing Lonapegsomatropin, is a human growth hormone indicated for the treatment of pediatric patients aged 1 year and older with growth failure due to inadequate secretion of endogenous growth hormone (GH).
How does Lonapegsomatropin work in the body?
Lonapegsomatropin, a prodrug of somatropin, undergoes autocleavage to release active somatropin. Somatropin then binds to the growth hormone receptor, leading to direct effects and insulin-like growth factor-1 (IGF-1) mediated indirect effects.
Is there any specific storage requirement for Skytrofa?
Skytrofa cartridges should be refrigerated at 36°F to 46°F (2°C to 8°C) in the outer carton to protect from light until the expiration date. They should not be frozen and can be stored at room temperature for up to 6 months under specific conditions.
What precautions should be taken during Skytrofa treatment?
Patients and healthcare providers should monitor for hypersensitivity reactions, increased risk of neoplasms, glucose intolerance, intracranial hypertension, fluid retention, and other potential adverse effects. Regular assessments and compliance checks are recommended.
Is Skytrofa safe during pregnancy and breastfeeding?
There are no available data on the use of Skytrofa in pregnant patients or its presence in human milk. The decision to use Skytrofa during pregnancy or breastfeeding should be made considering the potential benefits and risks.
How long should Skytrofa treatment be continued?
Skytrofa treatment should be continued as long as clinically indicated, and discontinuation should be considered once epiphyseal fusion has occurred. Contact your doctor for more information.
Is Skytrofa suitable for children under 1 year of age?
The safety and effectiveness of Skytrofa in children less than 1 year of age have not been established. Consultation with a healthcare professional is recommended for personalized guidance on treatment appropriateness.
What is the Skytrofa price in India?
When evaluating the price factors associated with Skytrofa in the Indian market, several components contribute, including import duties, taxes, exchange rates, currency variations, and the dynamics of demand and supply. For accurate and thorough pricing information, we suggest contacting our Patient Support Team via the provided contact details (+91) 93157 05373 or help@sansfro.com. Our team of experts is dedicated to providing specialized assistance, offering precise details to effectively address your inquiries.
How can I buy Skytrofa online?
If you are looking to buy Skytrofa online, a medication exclusively available in the US and Europe, we recommend contacting the Sansfro Health team or other reputable firms specializing in importing drugs from these regions. Ensuring a secure and reliable procurement process is crucial, and seeking guidance from experts is paramount. Sansfro Health is a reputable entity committed to facilitating access to authentic pharmaceuticals.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.