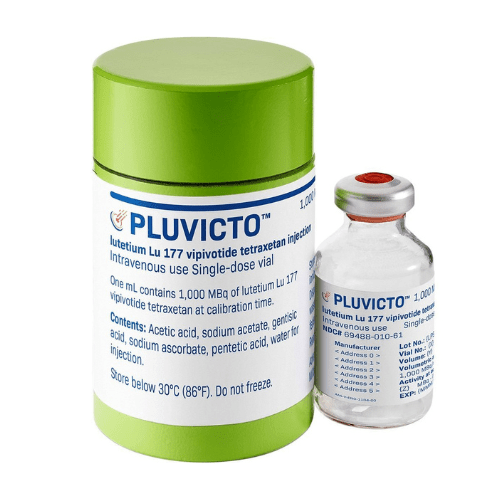
About Pluvicto (lutetium Lu 177 vipivotide tetraxetan)
- Pluvicto novartis received Food and Drug Administration (FDA) approval on On March 23, 2022, which belongs to the class of drugs known as radiopharmaceuticals.
- lutetium Lu 177 vipivotide tetraxetan is sold under the brand name Pluvicto.
- Lutetium (177Lu) vipivotide tetraxetan is a radiopharmaceutical medication prescribed for the treatment of metastatic castration-resistant prostate cancer (mCRPC) in patients with prostate-specific membrane antigen (PSMA) positivity. This targeted radioligand therapy is designed to address PSMA-positive mCRPC effectively.
- Prostate-specific membrane antigen (PSMA) is a glycoprotein primarily found in normal prostatic epithelial cells, prostate cancer, and endothelial cells. This membrane-bound protein is classified as a type II membrane protein and was initially identified through the murine monoclonal antibody (mAb) 7E11-C5.3.
- PSMA serves as a valuable target for both diagnostic and potential therapeutic purposes in the context of prostate cancer.
Strength:
The injection is available in a single-dose vial with a concentration of 1,000 mega becquerels per milliliter (27 millicuries per milliliter).
Recommended Dosage:
Pluvicto is administered intravenously using various methods. The recommended dosage may be delivered via injection using a disposable syringe equipped with a syringe shield, infusion using the gravity method (with or without an infusion pump), or infusion using the vial (with a peristaltic infusion pump). When administering a reduced dose, the syringe method (with or without a syringe pump) or the vial method (with a peristaltic infusion pump) should be employed. Prior to administration, the intravenous catheter used exclusively for Pluvicto should be flushed with ≥ 10 mL of 0.9% sterile sodium chloride solution to ensure patency and minimize the risk of extravasation.
The syringe method involves withdrawing an appropriate Pluvicto volume with a shielded syringe and administering it via intravenous push. For the gravity method, a short needle and a long needle are inserted into the Pluvicto vial, connecting to a catheter with sterile sodium chloride solution for transport. The peristaltic infusion pump method employs a short venting needle and a long needle connected to a catheter and peristaltic infusion pump, ensuring precise delivery. After reaching the desired radioactivity level, an intravenous flush of ≥ 10 mL of 0.9% sterile sodium chloride solution is recommended to complete the administration process.
- Patient Selection: Choose patients for Pluvicto treatment based on PSMA expression in tumors, assessed using LOCAMETZ® or an approved PSMA-11 imaging agent.
- Recommended Dosage: Administer 7.4 GBq (200 mCi) every 6 weeks for a maximum of 6 doses.
- To manage adverse reactions, consider dose adjustment, interruption, reduction, or permanent discontinuation. Temporary dose interruption, with a dosing interval extended up to 10 weeks, dose reduction, or permanent discontinuation may be necessary.
- If a treatment delay due to adverse reactions persists for more than 4 weeks, discontinuation of Pluvicto is recommended. A one-time 20% dose reduction to 5.9 GBq (160 mCi) is permissible, but re-escalation is not advised. Persistent or additional adverse reactions warrant discontinuation of Pluvicto.
Important:
For the preparation of Pluvicto, adhere to aseptic techniques and employ radiation shielding, utilizing tongs as necessary to minimize radiation exposure. Before administration, visually inspect the vial for particulate matter and discoloration under a shielded screen; discard the vial if any such abnormalities are detected. Avoid direct injection of the Pluvicto solution into any other intravenous solution. Before and following Pluvicto administration, use a properly calibrated dose calibrator to confirm the amount of radioactivity delivered to the patient. Dispose of any unused medicinal product or waste material in compliance with local and federal regulations.
Warnings & Precautions
- Minimize radiation exposure during and after Pluvicto therapy in accordance with institutional safety practices.
- Encourage patients to increase oral fluid intake and void frequently to reduce bladder radiation.
- Perform complete blood counts to monitor for myelosuppression, adjusting Pluvicto treatment based on severity.
- Advise patients to stay well-hydrated and urinate frequently to mitigate renal toxicity, adjusting Pluvicto administration as necessary.
- Caution male patients with reproductive partners about the potential for Pluvicto to cause fetal harm, emphasizing the use of effective contraception. Pluvicto may cause infertility in male. Hence, inform patients about the possibility of temporary or permanent infertility associated with Pluvicto.
Common Pluvicto Side Effects:
- Fatigue (tiredness)
- Dry mouth
- Nausea
- Low red blood cell count (anemia)
- Loss of appetite
- Changes in bowel movements (constipation or diarrhea)
- Vomiting
- Low blood platelet count
- Urinary tract infection (UTI)
- Weight loss
- Abdominal pain
- Decreased lymphocytes
- Decreased calcium
- Decreased sodium
Use in Specific Population
- The safety and efficacy of Pluvicto have not been verified in females.
- There is no available data on the presence of lutetium Lu 177 vipivotide tetraxetan in human milk or its impact on breastfeeding infants or milk production.
- Given its mechanism of action, male patients with female partners of reproductive potential should use effective contraception throughout Pluvicto treatment and for 14 weeks after the last dose.
- The safety and efficacy of Pluvicto in pediatric patients remain unestablished.
- No discernible differences in effectiveness were noted between patients aged 75 years and older and younger patients.
- Patients with mild (baseline creatinine clearance [CLcr] 60 to 89 mL/min by Cockcroft-Gault) to moderate (CLcr 30 to 59 mL/min) renal impairment generally do not require dose adjustment; however, those with mild or moderate renal impairment may face an increased risk of toxicity.
Storage and Handling
- Keep Pluvicto stored below 30°C (86°F) and avoid freezing.
- Store it in the original package to shield it from ionizing radiation, using lead shielding as necessary.
- Adhere to local and federal regulations regarding radioactive materials when storing Pluvicto.
- Refrain from using Pluvicto after the expiration date and time specified on the label.
- Dispose of any unused medication or waste materials in compliance with local and federal laws.
- Ensure proper storage to maintain the efficacy of the medication.
- Protect the medicine from direct light exposure, and avoid freezing it.
- Keep Pluvicto out of reach of pets and children, emphasizing the importance of secure and responsible storage practices.
Our process for procuring medications follows a well-defined four-step methodology, ensuring a streamlined experience:
- Inquiry: When you seek information about a medication, our Named Access Program Support team responds promptly, reaching out to assist you within 24 hours.
- Validation: Sansfro guarantees the availability and endorsement of medicines, especially for patients seeking medications not readily accessible in their home countries. We meticulously authenticate prescriptions and medical details for precision and compliance.
- Acquisition: After successful validation, our team taps into its network of suppliers to acquire the necessary medication. We negotiate advantageous quotes and supervise the seamless processing of orders.
- Secure Delivery: Upon finalizing the quote, we efficiently coordinate the secure delivery of your medication. Our logistics specialists are available for consignment tracking. To uphold the integrity of medication provision, we rigorously adhere to Standard Operating Procedures in the Named Access Program industry.
To facilitate the importation of medication, patients must furnish the following documentation:
- A valid prescription copy.
- Identification records.
- Information about the primary healthcare provider.
- Current residence address.
Once all essential documents are provided, the Sansfro team promptly initiates the import license application process. This license is a critical prerequisite for procuring the necessary medication upon government approval.
No news found.
About Sansfro
Sansfro is committed to bridging the healthcare gap. Our purpose is to bring hope and healing to patients suffering from rare diseases by linking them with life-saving treatments through our Named Patient Program. Join us in our quest for a better and healthier world.
Know More...FAQ'S
What is the active ingredient of Pluvicto?
The active ingredient of Pluvicto is called is lutetium Lu 177 vipivotide tetraxetan. It is belongs to the class of drugs known as radiopharmaceuticals.
What is Pluvicto used for?
Pluvicto is a radioligand therapeutic agent indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have undergone prior treatment with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy.
How should patients be selected for Pluvicto prostate cancer treatment?
The selection of patients for treatment should be conducted through the utilization of Locametz or an officially approved PSMA-11 imaging agent. This selection should be based on the evaluation of PSMA expression in tumors.
What is the recommended dosage for Pluvicto?
The recommended dosage for Pluvicto is to administer 7.4 GBq (200 mCi) every 6 weeks for up to 6 doses.
Are there any contraindications for Pluvicto?
No, there are no contraindications listed for Pluvicto.
What are the key warnings and precautions associated with Pluvicto?
- Minimize radiation exposure during and after treatment, following good radiation safety practices.
- Monitor for myelosuppression through complete blood counts, with dose adjustments or discontinuation based on severity.
- Address renal toxicity by advising patients to stay well-hydrated and monitoring kidney function; adjust or discontinue Pluvicto based on severity.
- Warn of potential embryo-fetal toxicity, necessitating effective contraception in male patients with female partners of reproductive potential.
- Highlight the possibility of Pluvicto causing temporary or permanent infertility.
What are the most common adverse reactions and laboratory abnormalities associated with Pluvicto?
Common adverse reactions comprise fatigue, dry mouth, nausea, anemia, decreased appetite, and constipation, while prevalent laboratory abnormalities include reduced lymphocytes, hemoglobin, leukocytes, platelets, calcium, and sodium levels.
What is the Pluvicto cost in India?
The price of Pluvicto in India is determined by product specifications. For accurate and detailed pricing information, we suggest contacting our Patient Support Team at (+91) 93157 05373 or help@sansfro.com.
How can I buy Pluvicto online?
When procuring Pluvicto online, available in the US and Europe, it is advisable to connect with the Sansfro Health team or reputable organizations specializing in medication importation from these regions to ensure a secure and reliable process. Seek guidance from experienced professionals, as Sansfro Health is a trustworthy company committed to providing access to genuine medications.

Dr Anchal Aryan
Medical counselor
Patient Stories
Word Wide Delivery:
India, Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, etc.